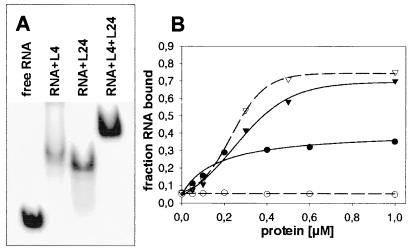
Figure 3.
Binding experiments with L4 and L24. (A) Band-shift assay with RNAL4-2 in the presence of the ribosomal proteins L4, L24, and L4 + L24, respectively: 7.5 μM RNA, 15 μM protein(s). The ionic conditions were 10 mM Hepes (pH 7.4), 4 mM MgCl2, 100 mM NH4Cl, and 4 mM β-mercaptoethanol. An equivalent experiment was performed with the shorter RNAL4-3 (53 nt), where the L4-rRNAL4-3 and L4-rRNAL4-3-L24 complexes migrated at similar positions. The band with the putative L4-rRNAL4-3-L24 complex was excised from the gel and checked for protein content by SDS-gel electrophoresis. Both L4 and L24 were detected, thus verifying the formation of the ternary complex. (B) Nitrocellulose filtration: Binding of L4 (triangles) and L24 (circles) to the fragment rRNAL4-3 (solid lines) and to the corresponding mutant G289U (broken lines).