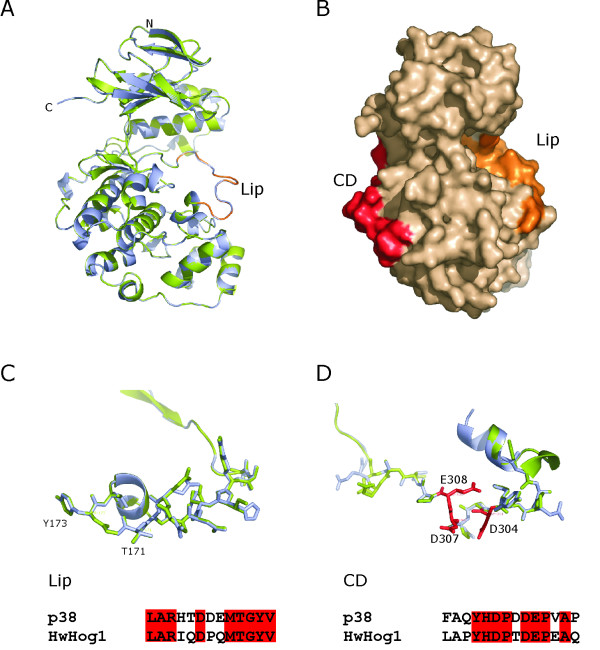
Figure 2.
Three-dimensional model of HwHog1. Superposition of HwHog1 (green) and murine p38 (blue). (A) Ribbon diagram of the conservation of secondary structure elements. The position of the phosphorylation Lip region of HwHog1 is shown (orange). (B) Surface view of the HwHog1 protein. The conserved CD domain (red) and the phosphorylation Lip (orange) are exposed on the surface at opposite sides of the protein. (C) The architecture of the phosphorylation Lip, and (D) the CD domain amino-acid residues are represented by sticks. The positions of the phosphorylation site residues Thr171 and Tyr173 are marked. The negatively charged Asp304, Asp307 and Glu308 amino acids in the CD domain of HwHog1 are denoted by red sticks. The amino-acid residue conservation between the HwHog1 and p38 sequences is denoted by red boxes. The plots were created with the PyMOL programme (version 0.97 [35]).