Abstract
Childhood spinal cord astrocytomas are rare diseases, and their management is controversial. We report here our successful experience using irinotecan and cisplatin in three consecutive infants with progressing intramedullary astrocytomas. The first patient was a 16-month-old girl who presented with a grade III intramedullary astrocytoma that rapidly progressed after surgery and adjuvant chemotherapy. Weekly irinotecan (50 mg/m2) and cisplatin (30 mg/m2) for four consecutive weeks (one cycle) for a total of four cycles (I/C regimen) was used in order to avoid or delay radiotherapy. Radiological complete remission was achieved 10 months after completion of therapy, and 3.5 years after diagnosis the patient remains disease free. The second patient was a 19-month-old boy with a C3-T4 grade II intramedullary astrocytoma who received up-front vincristine and carboplatin for two months but remained clinically symptomatic. A follow-up MRI showed a larger tumor, and the patient was switched to the I/C regimen. A marked clinical improvement occurred after the first cycle, and MRI showed a very good partial remission at the end of therapy. At 16 months after diagnosis, the patient remains disease free. The third patient was a 10-month-old girl with a C2-T3 grade II intramedullary astrocytoma. She presented with severe pain that became steroid dependent during the month she was treated with the vincristine-carboplatin regimen. When she was switched to the I/C regimen, the clinical symptoms responded within days. MRI at the end of therapy showed a significant reduction in tumor size, and one year after diagnosis the patient remains symptom free. Using this I/C regimen for childhood intramedullary astrocytoma, we obtained remarkable clinicoradiological responses while avoiding the use of radiotherapy.
Keywords: chemotherapy, childhood brain tumor, cisplatin, intramedullary astrocytoma, irinotecan, primary intramedullary spinal cord neoplasms
Spinal cord tumors (SCTs)2 are rare, especially in children younger than three years of age, and represent 4%–10% of all pediatric tumors of the CNS. The most frequent primary location is the cervical spine, followed by the thoracic spine, although SCTs may present as holocord tumors (Lowis et al., 1998). Of all SCTs in children, 60% are astrocytomas, usually large tumors occupying more than three cord levels, followed by ependymomas (Nadkarni and Rekate, 1999).
Management of intramedullary SCTs in children is controversial. Because of the rarity of such tumors, few appropriate studies have been conducted to evaluate the effect of different treatments. There is general agreement that high-grade astrocytomas require multidisciplinary intervention because their gross total resection is difficult due to their infiltrative behavior. Adjuvant therapies for high-grade spinal cord astrocytomas have been analyzed together with those for intracranial astrocytomas, and the overall outcome has been equally poor. Effective chemotherapy regimens can be found (Houten and Cooper, 2000; Pollack, 2004). For instance, the 8 in 1 protocol (Children’s Cancer Group 945) was designed specifically for the treatment of patients with high-grade astrocytomas, and the results showed that such a regimen was clearly suboptimal for SCTs (Allen et al., 1998). For children younger than three years of age with spinal or brain high-grade astrocytomas, the majority of investigators have used adjuvant chemotherapy regimens known as baby protocols after surgical resection to delay or avoid radiation therapy as much as possible. Baby protocols include combinations of drugs such as cisplatin, carboplatin, methotrexate, etoposide, cyclophosphamide, and vincristine (Allen et al., 1998; Duffner et al., 1993, 1999; Geyer et al., 1995). The results of such regimens, however, are quite poor (Geyer et al., 1995; Massimino et al., 2000; Pollack, 2004).
Optimal management of low-grade spinal cord astrocytomas in children is open for debate (Pollack, 2004). Generally, after incomplete surgery, adjuvant chemotherapy is recommended for symptomatic children younger than three years, and radiotherapy is reserved for older children and cases of relapse (reviewed by Gnekow et al., 2004).
Irinotecan is a water-soluble camptothecin pro-drug that is converted to an active metabolite, SN-38, a camptothecin analogue that is a topoisomerase I poison 100 times more potent than the pro-drug. The camptothecin analogues are cytotoxic agents that bind to and stabilize the intranuclear enzyme topoisomerase I, resulting in enzyme-linked DNA breaks that cannot be religated as long as the drug is present (Hertzberg et al., 1989). The results of a number of phase I and phase II clinical trials in children treated with irinotecan have been published and more are ongoing (Blaney et al., 2001; Bomgaars et al., 2006; Cosetti et al., 2002; Furman et al., 1999; Houghton and Santana, 2002; Kushner et al., 2005; Turner et al., 2002; Vassal et al., 2003). Investigations of scheduling and mechanisms of resistance have found that not all camptothecin analogues are equally effective for all tumors and that appropriate combination regimens are more effective than irinotecan alone (Bomgaars et al., 2001). The antitumor activity of irinotecan has been shown to increase after sequential administration of an alkylating agent such as carmustine or temozolomide (Coggins et al., 1998; Houghton and Santana, 2002; Pourquier et al., 2001). The most frequently used schedule of administration involves weekly doses of irinotecan and cisplatin, and this same strategy reported by Souid et al. (2003) in a phase I study in children with refractory solid tumors showed that it is well tolerated. When used as a single agent, irinotecan has shown objective antitumor responses in a variety of childhood tumors, including rhabdomyosarcoma (Blaney et al., 2001; Cosetti et al., 2002; Furman et al., 1999), neuroblastoma (Cosetti et al., 2002; Kushner et al., 2005; Vassal et al., 2003), and CNS tumors (Turner et al., 2002; Vassal et al., 2003). A report by Turner et al. (2002) showed a response rate of more than 40% in children with intracranial malignant glioma when a dose of 125 mg/m2 per week for four weeks was used.
Cisplatin enters the cell and produces cationic species that bind to the DNA, altering its structure, affecting its ability to act as a template in transcription, and eventually promoting cell death by apoptosis. The primary toxic effects are nephrotoxicity, peripheral neuropathy, and ototoxicity for cisplatin and myelosuppression and diarrhea for irinotecan. Cisplatin has a broad range of antitumor activity and is used as a frontline therapy in a variety of pediatric solid tumors, including astrocytomas. Using cisplatin in combination with etoposide, Massimino et al. (2002) found a remarkable 70% response rate in children with progressing low-grade CNS astrocytomas. The different mechanisms of action and the lack of overlapping toxicities of the irinotecan-cisplatin combination have prompted many clinical trials in adults, showing that the combination is both safe and effective (Kobayashi et al., 1998; Masuda et al., 1994; Okamoto et al., 1998; Shinkai et al., 1994).
Taking into account the antineoplastic effect observed in astrocytomas when combinations containing either cisplatin (Massimino et al., 2002) or irinotecan (Turner et al., 2002) were used, we explored the possibility of treating very young patients who had progressing tumors after conventional chemotherapy with regimens combining cisplatin with irinotecan, with the aim of avoiding radiation therapy. Here we report three consecutive patients with clinically and radiologically progressing intramedullary astrocytomas despite initial surgical resection and carboplatin-based adjuvant chemotherapy. Treatment with weekly irinotecan and cisplatin provided objective and stable responses, which enabled radiation therapy to be avoided. Because therapy for children with these tumors is frequently unsuccessful, the observation of promising activity and low toxicity of new combinations of agents with novel mechanisms of action brings new hope for treatment success.
Case Studies
Case 1
A previously healthy, 16-month-old girl presented with a two-month history of gait disturbance. She had neither pain nor sphincter dysfunction. Her physical examination showed a hemiparetic and wide-based gait with abolition of all deep-tendon reflexes in her left lower limb. Findings for the rest of her physical examination were normal. MRI of the spine revealed a cystic intramedullary tumor 3 cm long × 1 cm wide, from T10 to L1. The tumor did not enhance with gadolinium (Fig. 1A). Neurological deficits of the disease, before surgery, were partial paralysis of the left lower limb with equinovarus foot and left hemiparetic gait; otherwise, the patient had good sphincter control. These deficits correspond to a functional grade II on the McCormick scale (McCormick et al., 1990).
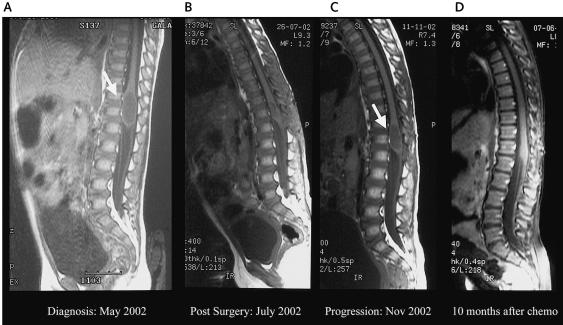
Fig. 1.
MRIs of the spine of patient 1. (A) MRI at diagnosis showing a cystic intramedullary tumor, not enhanced by gadolinium, 3 cm long × 1 cm wide, from T10 to L1. (B) Postoperative MRI showing a residual solid tumor lesion, 20 × 7 × 3 mm, in the spinal cord cone, with an associated 3 × 4-mm cyst. (C) MRI from three months into treatment, showing a larger cystic lesion, 24 × 12 × 12 mm, hyperintense compared with the CSF, with no gadolinium enhancement. (D) MRI from 10 months after the conclusion of the irinotecan and cisplatin therapy, showing a normal spinal cone.
A T10-L1 laminectomy with partial tumor resection was performed. The tumor was poorly capsulated, precluding complete resection. Histology showed a tumor composed of highly proliferative astrocytic cells, with moderate atypias, few mitosis figures, and no necrosis or vascular hyperplasia. The entire tumor was homogeneously positive for the glial fibrillary protein, and 50%–60% of the tumor cells were positive for Ki-67. The final diagnosis was compatible with a grade III intramedullary astrocytoma. Results of cerebrospinal fluid (CSF) cytology for tumor cells were negative. The postoperative MRI showed a residual solid tumor lesion 20 × 7 × 3 mm in the spinal cord cone with an associated 3 × 4-mm cyst (Fig. 1B).
Because of the age of the patient, the histology, and the residual tumor, adjuvant chemotherapy was initiated following the Sociedad Española de Oncología Pediatrica (SEOP) baby protocol that uses sequential courses of two-drug combinations, including vincristine, cisplatin, etoposide, cyclophosphamide, methotrexate, and carboplatin. After three months of treatment, a follow-up MRI showed a larger cystic lesion, 24 × 12 × 12 mm, hyperintense compared with the CSF, with no gadolinium enhancement (Fig. 1C), suggesting tumor progression. At that point, surgery was not recommended. Because the patient remained neurologically stable, we decided to test new chemotherapy regimens before using radiation therapy.
We used weekly irinotecan (50 mg/m2) and cisplatin (30 mg/m2) for four consecutive weeks (one cycle), with a week off between cycles, for a total of four cycles (I/C regimen), approximately 20 weeks of therapy. We devised the schema from reported experiences (Souid et al., 2003) and the Children’s Oncology Group protocol P9970. After two cycles, a follow-up MRI showed disease stabilization with the cone lesion measuring 24 × 12 × 12 mm. After the fourth cycle, chemotherapy was stopped, and MRI scans were performed every two months to document evolution. Six months after the conclusion of therapy, the MRI showed the first decrease in tumor size (to 20 × 7 × 5 mm). Ten months after completion of chemotherapy, the MRI demonstrated a normal-sized spinal cone with no contrast uptakes and a 4-mm residual cystic lesion (Fig. 1D). To date (now four years after diagnosis), the patient has remained in continued remission, and results of serially performed renal function and audiometric tests have been within normal limits.
Case 2
A 19-month-old boy presented with a one-month history of cervical pain and torticollis. He was irritable, and cervical spine manipulation was extremely painful. MRI of the spine showed a large intramedullary tumor, 5.5 cm long × 1.5 cm wide, from C4 to T4 (Fig. 2A). The spinal cord was uniformly enlarged, with blockage of the subarachnoidal perimedullary space. A laminotomy with partial resection was performed due to the infiltrative aspect of the tumor. Histology was compatible with a fibrillary, grade II intramedullary astrocytoma. CSF cytology results for tumor cells were negative.
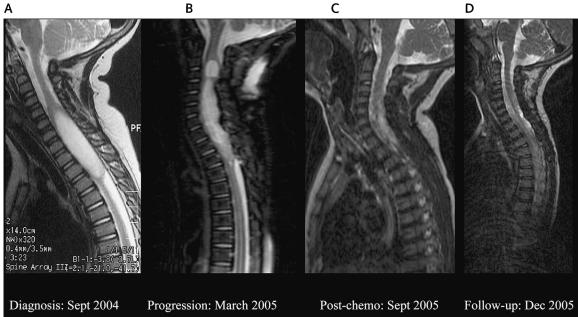
Fig. 2.
MRIs of the spine of patient 2. (A) MRI at diagnosis, showing an intramedullary tumor, 5.5 cm long × 1.5 cm wide, from C4 to T4. (B) Control MRI showing a larger tumor from C3 to T3, hyperintense compared with the CSF, with no gadolinium enhancement, suggesting tumor progression. (C and D) Two subsequent posttherapy MRIs, showing a continued decrease in the tumor size and progressive normalization of the cervical cord.
The patient was treated with dexamethasone and opioid-derived analgesia, and adjuvant chemotherapy was initiated following the Société Internationale d’Oncologie Pédiatrique (SIOP) 2001 protocol for treating low-grade astrocytomas. This regimen consisted of cycles of vincristine every week and carboplatin every three weeks for 10 weeks and then the combination of carboplatin and vincristine every four weeks for up to one year. Two months into therapy, the patient remained steroid dependent and still required opioid-derived analgesia to control his cervical pain. A follow-up MRI showed a larger tumor from C3 to T3, hyperintense compared with the CSF, with no gadolinium enhancement, suggesting tumor progression (Fig. 2B). We therefore changed the chemotherapy to the I/C regimen (March 2005). After three weeks of treatment, a marked improvement of his pain enabled all medications to be withdrawn. After two cycles (eight weeks of treatment), MRI showed disease stabilization, and, at the end of therapy (four cycles, June 2005), MRI showed a significant decrease in the cord enlargement, with no contrast enhancement (image not shown), suggestive of significant tumor regression. Two subsequent posttherapy MRIs (September and December 2005) showed a continued decrease in the tumor size and progressive normalization of the cervical cord (Fig. 2C and D). At 20 months after diagnosis, the patient remains symptom free and has regained normal activity accompanied by neurological recovery. Results for renal function and audiometric tests performed thus far have all been within normal limits.
Case 3
A 10-month-old girl presented with long-standing cervical pain and disuse of both arms. Spinal MRI revealed a 61-mm-long × 15-mm-wide intramedullary mass from C2 to T3. A laminotomy with biopsy was performed, and histopathology was compatible with a fibrillary, grade II intramedullary astrocytoma. The CSF cytology results for tumor cells were negative.
The patient was initially treated (March 2005) according to the SIOP protocol. One month into therapy, however, clinical signs worsened, with increased pain and weakness of both arms. A new MRI showed no radiological signs of response, and chemotherapy was switched to the I/C regimen (Fig. 3A). After one cycle, pain was controlled, and the steroids were withdrawn. The patient remained asymptomatic throughout therapy, and this response was accompanied by progressive neurological recovery and reuse of both arms. MRI at the end of therapy (four cycles, July 2005) showed a significant decrease in the tumor mass (C5-T2), with no contrast enhancement, suggestive of tumor regression (Fig. 3B). Two subsequent posttherapy MRIs (October 2005 and January 2006) showed a continued decrease in the tumor size and progressive normalization of the cervical cord (October 2005 [Fig. 3C]). At 12 months after diagnosis, the patient remains symptom free and is performing regular activities according to her age. Results of renal function and audiometric tests performed thus far have been normal.
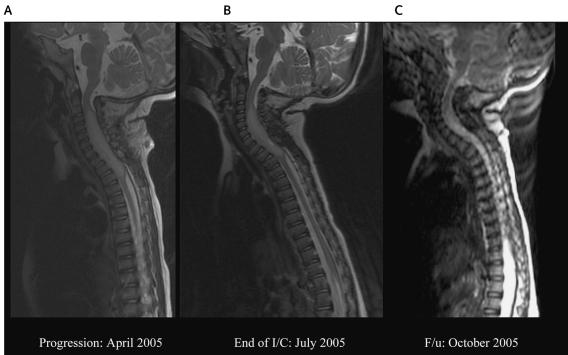
Fig. 3.
MRIs of the spine of patient 3. (A) MRI at diagnosis, showing an intramedullary tumor, 61 mm long × 15 mm wide, from C2 to T3. (B) MRI at the end of irinotecan and cisplatin (I/C) chemotherapy, showing a significant decrease in the tumor mass (from C5 to T2), with no contrast enhancement, suggestive of tumor regression. (C) A follow-up (F/u) MRI showing a continued decrease in the tumor size and progressive normalization of the cervical cord.
Discussion
Intracranial and intramedullary astrocytomas show similar histological features and biological behavior, and they are classified according to the revised WHO grading system: grade I (pilocytic), grade II (protoplasmatic, fibrillary, or gemistocytic), grade III (anaplastic), and grade IV (glioblastoma multiforme) (Kleihues et al., 2000). In adult studies, median five-year survival rates have been about 76% for patients with grade I tumors and 68% for those with grade II tumors; for patients with grade III and grade IV tumors, median survival time is only 15 months (Innocenzi et al., 1997). Studies in children have found five-year progression-free survival rates of 78% for low-grade astrocytomas (grades I and II) and 30% for high-grade astrocytomas (grades III and IV) (Constantini et al., 2000). The higher prevalence of grade I astrocytomas in the pediatric series could explain the more favorable prognosis overall, compared with findings for adults (Bouffet et al., 1998; Constantini et al., 2000; Raco et al., 2005). In addition to the histological grade, the prognosis for patients with intramedullary SCTs has been related to the patients’ preoperative neurological condition (Bouffet et al., 1998; Constantini et al., 2000; Hassall et al., 2001; Lowis et al., 1998; McCormick et al., 1990; Nadkarni and Rekate, 1999; Raco et al., 2005). Patients presenting with pain and decreased motor function have a poorer prognosis than do asymptomatic patients or those presenting with scoliosis (Townsend et al., 2004). Our three patients presented with motor paralysis and/or difficult-to-control pain.
Surgery alone may be curative for patients with low-grade lesions, and complete resection had a favorable effect in a series of pediatric cases with a variety of SCTs, especially ependymomas (Goh et al., 1997). WHO grade I tumors are well circumscribed and thus potentially amenable to cure with surgery alone. In contrast, WHO grade II–IV tumors are diffusely infiltrative and infrequently cured with surgery alone. However, the effect of surgery on different pediatric CNS malignancies as well on SCTs is well recognized (Constantini et al., 2000; Jallo et al., 2003; Raco et al., 2005). In different series, including all kinds of pediatric SCTs, radical excision achieved longer event-free survival times than did palliative/subtotal resection or radiotherapy alone (Constantini et al., 2000; Jallo et al., 2003; Nadkarni and Rekate, 1999; Raco et al., 2005). Nevertheless, given that aggressive surgery increases the risk of severe neurological morbidity and spinal deformity in patients with expected long-term survival, some investigators advocate a conservative surgical policy followed by adjuvant therapy for low-grade tumors (Doireau et al., 1999; Raco et al., 2005). For high-grade astrocytomas, surgery is usually incomplete, and there is agreement that, because of the aggressive behavior of such tumors, their management requires the use of adjuvant therapies. Because of our recent experience with effective adjuvant chemotherapy for progressive spinal astrocytomas, we have shifted our surgical approach from up-front radical tumor resection with intraoperative somatosensory and motor-evoked potential monitoring toward conservative surgery, followed by chemotherapy for all patients. In addition, we consider surgery as a rescue procedure in cases in which adjuvant therapy fails to control the disease.
Chemotherapy side effects appear less severe when compared with the potential damage induced by radiotherapy in the immature CNS of young children. There are several reports regarding the effectiveness of chemotherapy for spinal cord astrocytomas in children (Allen et al., 1998; Bouffet et al., 1998; Doireau et al., 1999; Foreman et al., 1998; Hassall et al., 2001; Lowis et al., 1998; Townsend et al., 2004). For low-grade, unresectable, progressing lesions, carboplatin-based therapy has shown an effect on survival for patients with brain and spinal cord astrocytomas (Doireau et al., 1999; Gururangan et al., 2002; Hassall et al., 2001; Houten and Cooper, 2000). The benefit of adjuvant chemotherapy seems to be most clearly shown in patients younger than three years, whose developing CNS is at most risk from the damage of radiotherapy (Packer et al., 1997). All of our patients were younger than three years, which may help explain the responses observed.
One of the most interesting observations in this study was the fast clinical improvement of patients 2 and 3 after only a few days of I/C therapy. Remarkable responses, with complete and prolonged pain disappearance, have previously been recognized by Doireau et al. (1999) as a sign of chemotherapy response. In our experience, such responses were not observed with carboplatin-based regimens. The worsening clinical signs of patients 2 and 3 and the radiological tumor regrowth in patient 1 while on carboplatin-based regimens argue against a potential effect of carboplatin-based regimens to explain the final response. According to our results, as well as the experience reported by Massimino et al. (2002) using cisplatin-based chemotherapy, we assume that at least part of the demonstrated effectiveness comes from the potentiated action of cisplatin. Because irinotecan itself has shown antiglioma activity at higher dosages (Turner et al., 2002), we hypothesize that this combination of drugs acts synergistically to produce a rapid clinical effect. Regarding pain control, a hypothetical anti-inflammatory and antiangiogenic effect of the I/C chemotherapy can be postulated. A recent study by Coull et al. (2005) showed that a continuous microglial-neuronal signaling is required to maintain neuropathic pain. Because glioma-infiltrating microglia is a major source of prostaglandin production through the cyclo-oxygenase 2 pathway, it could be speculated that the I/C chemotherapy had a direct anti-inflammatory effect on the tumor-infiltrated microglia. However, the rapid clinical response, as well as the relatively rapid radiological response (within six months), could also be caused by a direct antiangiogenic effect of irinotecan, because irinotecan has shown antiangiogenic activity in addition to its antiglioma effect (Kamiyama et al., 2005). Furthermore, continuous use of irinotecan as metronomic chemotherapy may represent an alternative treatment for low-grade astrocytoma.
The I/C regimen is relatively easy to administer in an outpatient setting and much shorter (a total of 20 weeks) than carboplatin-based protocols (usually one year or more). It was well tolerated, with no delay owing to toxicity between cycles. Serial audiological and renal function evaluations were conducted, and thus far neither ototoxicity nor renal function abnormalities have been documented. Median follow-up time has been short, however, and our patients continue to be monitored closely. Gastrointestinal tolerance during treatment was excellent in these patients, and antidiarrheal medicines were not needed.
Radiation therapy has played a limited role in the management of intramedullary astrocytoma in children, mainly because of the potential associated side effects, including growth retardation, endocrine dysfunction, radionecrosis, and vasculopathy. Postoperative radiation therapy has shown some benefit for patients with incompletely resected low-grade astrocytomas (Isaacson, 2000). However, there is a significant risk of infield recurrence, as well as damage to the bone and myelitis (Clayton and Shalet, 1991; Koshy et al., 2004; Townsend et al., 2004). After surgery, most authors recommend wait-and-see approaches for asymptomatic, low-grade astrocytomas and reserve radiation therapy for progression or recurrence. For high-grade astrocytomas, radiation therapy seemed unavoidable in most instances (Isaacson, 2000; Jallo et al., 2003). However, the prospective experience reported by the French Society of Paediatric Oncology (Doireau et al., 1999) suggested that adjuvant chemotherapy was in fact effective in those patients with recurrent or progressive unresectable SCTs, including high-grade lesions. Our first patient’s lesion had high-grade histological features, and the growth rate of the tumor after surgery confirmed the suspicion of a high-grade intramedullary astrocytoma. The disappearance of the tumor with use of the I/C regimen suggests that at least some high-grade tumors can be managed with chemotherapy alone, enabling radiotherapy to be avoided. To ascertain which high-grade astrocytomas may be curable with chemotherapy alone, and what new combination of drugs may be more effective, deserves further investigation.
In conclusion, despite a relatively short follow-up, our results suggest that a short but intensive regimen of irinotecan and cisplatin may be an effective adjuvant therapy for the treatment of progressing spinal cord astrocytomas in children. The potential benefit of this chemotherapy combination deserves further evaluation.
Acknowledgment
The authors thank the neurosurgical, neuroradiology, and pathology teams at Hospital Sant Joan de Déu, Barcelona, Spain, for their contributions.
Footnotes
Abbreviations used are as follows: CSF, cerebrospinal fluid; I/C, weekly irinotecan and cisplatin; SCT, spinal cord tumor; SIOP, Société Internationale d’Oncologie Pédiatrique.
References
- Allen JC, Aviner S, Yates AJ, Boyett JM, Cherlow JM, Turski PA, Epstein F, Finlay JL. Treatment of high-grade spinal cord astrocytoma of childhood with “8-in-1” chemotherapy and radiotherapy: A pilot study of CCG-945. Children’s Cancer Group. J Neurosurg. 1998;88:215–220. doi: 10.3171/jns.1998.88.2.0215. [DOI] [PubMed] [Google Scholar]
- Blaney S, Berg SL, Pratt C, Weitman S, Sullivan J, Luchtman-Jones L, Bernstein M. A phase I study of irinotecan in pediatric patients: A Pediatric Oncology Group study. Clin Cancer Res. 2001;7:32–37. [PubMed] [Google Scholar]
- Bomgaars L, Berg SL, Blaney SM. The development of camptothecin analogs in childhood cancers. Oncologist. 2001;6:506–516. doi: 10.1634/theoncologist.6-6-506. [DOI] [PubMed] [Google Scholar]
- Bomgaars L, Kerr J, Berg S, Kuttesch J, Klenke R, Blaney SM. A phase I study of irinotecan administered on a weekly schedule in pediatric patients. Pediatr Blood Cancer. 2006;46:50–55. doi: 10.1002/pbc.20355. [DOI] [PubMed] [Google Scholar]
- Bouffet E, Pierre-Kahn A, Marchal JC, Jouvet A, Kalifa C, Choux M, Dhellemmes P, Guerin J, Tremoulet M, Mottolese C. Prognostic factors in pediatric spinal cord astrocytoma. Cancer. 1998;83:2391–2399. doi: 10.1002/(sici)1097-0142(19981201)83:11<2391::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Clayton PE, Shalet SM. The evolution of spinal growth after irradiation. Clin Oncol (R Coll Radiol) 1991;3:220–222. doi: 10.1016/s0936-6555(05)80744-7. [DOI] [PubMed] [Google Scholar]
- Coggins CA, Elion GB, Houghton PJ, Hare CB, Keir S, Colvin OM, Bigner DD, Friedman HS. Enhancement of irinotecan (CPT-11) activity against central nervous system tumor xenografts by alkylating agents. Cancer Chemother Pharmacol. 1998;41:485–490. doi: 10.1007/s002800050771. [DOI] [PubMed] [Google Scholar]
- Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ. Radical excision of intramedullary spinal cord tumours: Surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg Spine. 2000;93:183–193. doi: 10.3171/spi.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- Cosetti M, Wexler LH, Calleja E, Trippett T, LaQuaglia M, Huvos AG, Gerald W, Healey JH, Meyers PA, Gorlick R. Irinotecan for pediatric solid tumors: The Memorial Sloan-Kettering experience. J Pediatr Hematol Oncol. 2002;24:101–105. doi: 10.1097/00043426-200202000-00009. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Doireau V, Grill J, Zerah M, Lellouch-Tubiana A, Couanet D, Chastagner P, Marchal JC, Grignon Y, Chouffai Z, Kalifa C. Chemotherapy for unresectable and recurrent intramedullary glial tumours in children. Brain Tumours Subcommittee of the French Society of Paediatric Oncology (SFOP) Br J Cancer. 1999;81:835–840. doi: 10.1038/sj.bjc.6690772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffner PK, Horowitz ME, Krischer JP, Krischer JP, Friedman HS, Burger PC, Cohen ME, Sanford RA, Mulhern RK, James HE, Freeman CR, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- Duffner PK, Horowitz ME, Krischer JP, Burger PC, Cohen ME, Sanford RA, Friedman HS, Kun LE. The treatment of malignant brain tumors in infants and very young children: An update of the Pediatric Oncology Group experience. Neuro-Oncology. 1999;1:152–161. doi: 10.1093/neuonc/1.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman NK, Hay TC, Handler M. Chemotherapy for spinal cord astrocytoma. Med Pediatr Oncol. 1998;30:311–312. doi: 10.1002/(sici)1096-911x(1998)30:5<311::aid-mpo12>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Furman WL, Stewart CF, Poquette CA, Pratt CB, Santana VM, Zamboni WC, Bowman LC, Ma MK, Hoffer FA, Meyer WH, Pappo AS, Walter AW, Houghton PJ. Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol. 1999;6:1815–1824. doi: 10.1200/JCO.1999.17.6.1815. [DOI] [PubMed] [Google Scholar]
- Geyer JR, Finlay JL, Boyett JM, Wisoff J, Yates A, Mao L, Packer RJ. Survival of infants with malignant astrocytomas: A report from the Children’s Cancer Group. Cancer. 1995;75:1045–1050. doi: 10.1002/1097-0142(19950215)75:4<1045::aid-cncr2820750422>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Gnekow, A.K., Packer, R.J., and Kortmann, R.D. (2004) Astrocytic tumors, low grade: Treatment considerations by primary site and tumor dissemination. In: Walker, D.A., Perilongo, G., Punt, J.A.G., and Taylor, R.E. (Eds.), Brain and Spinal Tumors of Childhood London: Arnold, pp. 259–276.
- Goh KY, Velasquez L, Epstein FJ. Pediatric intramedullary spinal cord tumors: Is surgery alone enough? Pediatr Neurosurg. 1997;27:34–39. doi: 10.1159/000121222. [DOI] [PubMed] [Google Scholar]
- Gururangan S, Cavazos CM, Ashley D, Herndon JE, II, Bruggers CS, Moghrabi A, Scarcella DL, Watral M, Tourt-Uhlig S, Reardon D, Friedman HS. Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol. 2002;20:2951–2958. doi: 10.1200/JCO.2002.12.008. [DOI] [PubMed] [Google Scholar]
- Hassall TE, Mitchell AE, Ashley DM. Carboplatin chemotherapy for progressive intramedullary spinal cord low-grade gliomas in children: Three case studies and a review of the literature. Neuro-Oncology. 2001;3:251–257. doi: 10.1093/neuonc/3.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg RP, Caranfa MJ, Holden KG, Jakas DR, Gallagher G, Mattern MR, Mong SM, Bartus JO, Johnson RK, Kingsbury WD. Modification of the hydroxy lactone ring of camptothecin: Inhibition of mammalian topoisomerase I and biological activity. J Med Chem. 1989;32:715–720. doi: 10.1021/jm00123a038. [DOI] [PubMed] [Google Scholar]
- Houghton PJ, Santana VM. Clinical trials using irinotecan. J Pediatr Hematol Oncol. 2002;24:84–85. doi: 10.1097/00043426-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Houten JK, Cooper PR. Spinal cord astrocytomas: Presentation, management and outcome. J Neurooncol. 2000;47:219–224. doi: 10.1023/a:1006466422143. [DOI] [PubMed] [Google Scholar]
- Innocenzi G, Salvati M, Cervoni L, Delfini R, Cantore G. Prognosis factors in intramedullary astrocytomas. Clin Neurol Neurosurg. 1997;99:1–5. doi: 10.1016/s0303-8467(96)00555-0. [DOI] [PubMed] [Google Scholar]
- Isaacson SR. Radiation therapy and the management of intramedullary spinal cord tumors. J Neurooncol. 2000;47:231–238. doi: 10.1023/a:1006470523052. [DOI] [PubMed] [Google Scholar]
- Jallo GI, Freed D, Epstein F. Intramedullary spinal cord tumors in children. Childs Nerv Syst. 2003;19:641–649. doi: 10.1007/s00381-003-0820-3. [DOI] [PubMed] [Google Scholar]
- Kamiyama H, Takano S, Tsuboi K, Matsumura A. Antiangiogenic effects of SN38 (active metabolite of irinotecan): Inhibition of hypoxia-inducible factor 1 alpha (HIF-1alpha)/vascular endothelial growth factor (VEGF) expression of glioma and growth of endothelial cells. J Cancer Res Clin Oncol. 2005;131:205–213. doi: 10.1007/s00432-004-0642-z. [DOI] [PubMed] [Google Scholar]
- Kleihues, P.D.R., Coons, S.W., and Burger, P.C. (2000) Astrocytic tumors. In: Kleihues, P., and Cavenee, W.B. (Eds.), Pathology and Genetics: Tumors of the Nervous System. World Health Organization Classification of Tumors Lyon, France: IARC, pp. 27–28.
- Kobayashi K, Shinbara A, Kamimura M, Takeda Y, Kudo K, Kabe J, Hibino S, Hino M, Shibuya M, Kudoh S. Irinotecan (CPT-11) in combination with weekly administration of cisplatin (CDDP) for non-small-cell lung cancer. Cancer Chemother Pharmacol. 1998;42:53–58. doi: 10.1007/s002800050784. [DOI] [PubMed] [Google Scholar]
- Koshy M, Paulino AC, Marcus RB, Jr, Ting J. The effect of an extended source-to-skin distance in the treatment of the spinal field in children receiving craniospinal irradiation. Med Dosim. 2004;29:7–10. doi: 10.1016/j.meddos.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Kushner BH, Kramer K, Modak S, Cheung NK. Five-day courses of irinotecan as palliative therapy for patients with neuroblastoma. Cancer. 2005;103:858–862. doi: 10.1002/cncr.20846. [DOI] [PubMed] [Google Scholar]
- Lowis SP, Pizer BL, Coakham H, Nelson RJ, Bouffet E. Chemotherapy for spinal cord astrocytoma: Can natural history be modified? Childs Nerv Syst. 1998;14:317–321. doi: 10.1007/s003810050233. [DOI] [PubMed] [Google Scholar]
- Massimino M, Gandola L, Cefalo G, Lasio G, Riva D, Fossati-Bellani F, Gianni MC, Luksch R, Tesoro-Tess JD, Lombardi F. Management of medulloblastoma and ependymoma in infants: A single-institution long-term retrospective report. Childs Nerv Syst. 2000;16:15–20. doi: 10.1007/PL00007279. [DOI] [PubMed] [Google Scholar]
- Massimino M, Spreafico F, Cefalo G, Riccardi R, Tesoro-Tess JD, Gandola L, Riva D, Ruggiero A, Valentini L, Mazza E, Genitori L, Di Rocco C, Navarria P, Casanova M, Ferrari A, Luksch R, Terenziani M, Balestrini MR, Colosimo C, Fossati-Bellani F. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20:4209–4216. doi: 10.1200/JCO.2002.08.087. [DOI] [PubMed] [Google Scholar]
- Masuda N, Fukuoka M, Kudoh S, Kusunoki Y, Matsui K, Nakagawa K, Hirashima T, Tamanoi M, Nitta T, Yana T, et al. Phase I study of irinotecan and cisplatin with granulocyte colony-stimulating factor support for advanced non-small-cell lung cancer. J Clin Oncol. 1994;12:90–96. doi: 10.1200/JCO.1994.12.1.90. [DOI] [PubMed] [Google Scholar]
- McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523–532. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- Nadkarni TD, Rekate HL. Pediatric intramedullary spinal cord tumors: Critical review of the literature. Childs Nerv Syst. 1999;15:17–28. doi: 10.1007/s003810050321. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Nagatomo A, Kunitoh H, Kunikane H, Watanabe K. A phase I clinical and pharmacologic study of a carboplatin and irinotecan regimen combined with recombinant human granulocyte-colony stimulating factor in the treatment of patients with advanced nonsmall cell lung carcinoma. Cancer. 1998;82:2166–2172. doi: 10.1002/(sici)1097-0142(19980601)82:11<2166::aid-cncr11>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Packer RJ, Ater J, Allen J, Phillips P, Geyer R, Nicholson HS, Jakacki R, Kurczynski E, Needle M, Finlay J, Reaman G, Boyett JM. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- Pollack IF. Intramedullary spinal cord astrocytomas in children. Pediatr Blood Cancer. 2004;43:617–618. doi: 10.1002/pbc.20129. [DOI] [PubMed] [Google Scholar]
- Pourquier P, Waltman JL, Urasaki Y, Loktionova NA, Pegg AE, Nitiss JL, Pommier Y. Topoisomerase I-mediated cytotoxicity of N-methyl-N’-nitro-N-nitrosoguanidine: Trapping of topoisomerase I by the O6-methylguanine. Cancer Res. 2001;61:53–58. [PubMed] [Google Scholar]
- Raco A, Esposito V, Lenzi J, Piccirilli M, Delfini R, Cantore G. Long term follow-up of intramedullary spinal cord tumors: A series of 202 cases. Neurosurgery. 2005;56:972–981. [PubMed] [Google Scholar]
- Shinkai T, Arioka H, Kunikane H, Eguchi K, Sasaki Y, Tamura T, Ohe Y, Oshita F, Nishio M, Karato A, et al. Phase I clinical trial of irinotecan (CPT-11), 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxy-camptothecin, and cisplatin in combination with fixed dose of vindesine in advanced non-small cell lung cancer. Cancer Res. 1994;54:2636–2642. [PubMed] [Google Scholar]
- Souid A, Dubowy RL, Blaney SM, Hershon L, Sullivan J, McLeod WD, Bernstein ML. Phase I clinical and pharmacologic study of weekly cisplatin and irinotecan combined with amifostine for refractory solid tumors. Clin Cancer Res. 2003;9:703–710. [PubMed] [Google Scholar]
- Townsend N, Handler M, Fleitz J, Foreman N. Intramedullary spinal cord astrocytomas in children. Pediatr Blood Cancer. 2004;43:629–632. doi: 10.1002/pbc.20082. [DOI] [PubMed] [Google Scholar]
- Turner CD, Gururangan S, Eastwood J, Bottom K, Watral M, Beason R, McLendon RE, Friedman AH, Tourt-Uhlig S, Miller LL, Friedman HS. Phase II study of irinotecan (CPT-11) in children with high-risk malignant brain tumors: The Duke experience. Neuro-Oncology. 2002;4:102–108. doi: 10.1093/neuonc/4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassal G, Doz F, Frappaz D, Imadalou K, Sicard E, Santos A, O’Quigley J, Germa C, Risse ML, Mignard D, Pein F. A phase I study of irinotecan as a 3-week schedule in children with refractory or recurrent solid tumors. J Clin Oncol. 2003;21:3844–3852. doi: 10.1200/JCO.2003.08.175. [DOI] [PubMed] [Google Scholar]