Abstract
Bartonella henselae causes cat scratch disease and the vasculoproliferative disorders bacillary angiomatosis and peliosis hepatis in humans. One of the best known pathogenicity factors of B. henselae is Bartonella adhesin A (BadA), which is modularly constructed, consisting of head, neck/stalk, and membrane anchor domains. BadA is important for the adhesion of B. henselae to extracellular-matrix proteins and endothelial cells (ECs). In this study, we analyzed different B. henselae strains for BadA expression, autoagglutination, fibronectin (Fn) binding, and adhesion to ECs. We found that the B. henselae strains Marseille, ATCC 49882, Freiburg 96BK3 (FR96BK3), FR96BK38, and G-5436 express BadA. Remarkably, BadA expression was lacking in a B. henselae ATCC 49882 variant, in strains ATCC 49793 and Berlin-1, and in the majority of bacteria of strain Berlin-2. Adherence of B. henselae to ECs and Fn reliably correlated with BadA expression. badA was present in all tested strains, although the length of the gene varied significantly due to length variations of the stalk region. Sequencing of the promoter, head, and membrane anchor regions revealed only minor differences that did not correlate with BadA expression, apart from strain Berlin-1, in which a 1-bp deletion led to a frameshift in the head region of BadA. Our data suggest that, apart from the identified genetic modifications (frameshift deletion and recombination), other so-far-unknown regulatory mechanisms influence BadA expression. Because of variations between and within different B. henselae isolates, BadA expression should be analyzed before performing infection experiments with B. henselae.
Bartonella henselae is a gram-negative, facultatively intracellular, fastidious, and slow-growing bacterium. Cats are the reservoir host of the bacteria, and transmission to humans occurs through cat scratches or cat fleas (8). Usually, in immunocompetent patients, a B. henselae infection results in cat scratch disease, an often self-limiting disease characterized by lymphadenopathy. In immunocompromised patients, B. henselae infections can cause tumorous proliferations of endothelial cells (ECs) in the skin or inner organs, which are called bacillary angiomatosis or peliosis hepatis, respectively (2). The ability to induce vasculoproliferations is a unique feature of Bartonella spp. Both in vitro and in vivo, B. henselae infections lead to the activation of hypoxia-inducible factor 1 (HIF-1), the key transcription factor involved in angiogenesis, and to the secretion of vasculoproliferative cytokines, e.g., vascular endothelial growth factor (VEGF) (15, 17).
One important pathogenicity factor of B. henselae is Bartonella adhesin A (BadA) (29), originally described as a “type IV-like pilus” (4). The 340-kDa BadA (monomer) consists of an N-terminal head region; a long, highly repetitive stalk; and a C-terminal membrane anchor (29). Together with Yersinia adhesin A (YadA) of Yersinia enterocolitica, Haemophilus influenzae adhesin (Hia) and Haemophilus surface fibrils (Hsf) of H. influenzae, and ubiquitous surface protein A (UspA) of Moraxella catarrhalis, BadA belongs to the class of trimeric autotransporter adhesins (TAAs), which all share similar modular architectures. The membrane anchor distinguishes TAAs from classical monomeric autotransporters; it is a highly conserved element, present throughout the protein family (14, 22), that has been shown to form trimers (19, 26, 34).
BadA is crucial for the adhesion of B. henselae to host cells and extracellular-matrix proteins (fibronectin [Fn] and collagens). It is also important for the activation of HIF-1 and the induction of VEGF secretion in infected host cells (17, 29). Variably expressed outer membrane proteins (Vomps) of Bartonella quintana (37) and Bartonella repeat protein A (BrpA) of Bartonella vinsonii (12), which are all highly homologous to BadA, are also members of the TAA family (22).
BadA expression is lost after multiple passaging of the bacteria in vitro. This phenomenon has been described as “phase variation” (4), but the underlying mechanism of gene regulation is not known. In addition, in many studies, the exact passage number of the bacteria is unfortunately not stated, and moreover, it is not clear whether these strains express BadA. This is somewhat problematic, as a lack of BadA expression might greatly influence the outcome of infection experiments with B. henselae. The only BadA-negative B. henselae strain that has been characterized functionally and genetically so far is a highly passaged variant of B. henselae Marseille (17), in which a deletion of the 5′ end of badA and a large region upstream of the gene occurred (29).
Here, we analyzed 10 B. henselae strains (human and cat isolates from different geographical regions) that are widely used in infection experiments for (i) BadA expression, by immunofluorescence and Western blotting; (ii) BadA-dependent autoagglutination; and (iii) the correlation between BadA expression and the ability of the strains to infect ECs. We found that adherence to ECs and Fn reliably correlated with the expression of BadA, confirming the importance of BadA in host cell infection. Also, as the regulation of BadA expression is not yet understood, the genes and the putative promoter regions were analyzed by PCRs and partial sequencing, showing that badA is present in all tested strains, although it is not expressed in several isolates. In one strain (Berlin-1), a frameshift deletion in the head region of badA correlated with a lack of BadA expression, whereas in three BadA-negative isolates (an ATCC 49882 variant, ATCC 49793, and Berlin-2), no explanation based on the analysis of the promoter region, the head, and the membrane anchor domain can be given.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study are summarized in Table 1. All B. henselae strains were grown on Columbia blood agar (Becton Dickinson, Heidelberg, Germany) in a humidified atmosphere at 37°C and 5% CO2. Except for B. henselae Marseille wild type and B. henselae Marseille variant strains, the passage numbers of the strains are not known.
TABLE 1.
B. henselae strains used in this study
| Designation | Other designation | Passage no. | Characteristic(s) | Reference or source |
|---|---|---|---|---|
| Marseille wild type | URLLY-8 | <10 | Human isolate from a cat scratch disease patient; BadA+ | 11; D. Raoult, Marseille, France |
| Marseille variant | URLLY-8 | >50 | BadA-negative variant of strain Marseille; extensively passaged; originally described as “pilus” negative | 17 |
| ATCC 49882 | Houston-1 | Unknown | Human isolate from blood of an AIDS patient | 1, 28 |
| ATCC 49882 variant | Houston-1 | Unknown | Variant of ATCC 49882; laboratory strain | A. Sander, Freiburg, Germany |
| ATCC 49793 | 87-66 Oklahoma | Unknown | Human isolate from blood | 4, 33 |
| FR96BK3 | Unknown | Cat isolate, Freiburg, Germany | 30 | |
| FR96BK38 | Unknown | Cat isolate, Freiburg, Germany | 30 | |
| Berlin-1 | Unknown | Human isolate from a bacillary angiomatosis patient, Berlin, Germany | 3 | |
| Berlin-2 | Unknown | Cat isolate, Berlin, Germany | 3 | |
| G-5436 | Houston-1 | Unknown | Human isolate from blood | 35, 36; CDC |
Determination of autoagglutination.
B. henselae strains were harvested from agar plates after 5 days of growth and resuspended in phosphate-buffered saline (PBS). The optical density at 600 nm (OD600) was adjusted to 2.0 (∼1 × 109 bacteria per ml), and bacteria were used at a concentration of ∼2 × 108 per ml. Three milliliters of each suspension was added to a plastic tube and incubated at 37°C with 5% CO2 in a humidified atmosphere. After 60 min, 10 μl of the suspension was taken from the bottom of each tube and transferred to a glass slide, air dried, and fixed with 3.75% PBS-buffered paraformaldehyde (PFA). After being washed with PBS, the bacteria were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (1 μg/ml dissolved in PBS). Autoagglutination was evaluated by confocal laser scanning microscopy (CLSM) (see below). Images were digitally processed with Photoshop 6.0 (Adobe Systems).
Culture and infection of endothelial cells.
Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial cell growth medium (PromoCell, Heidelberg, Germany), and infection experiments were performed in endothelial cell basal medium (PromoCell) as described previously (16). Briefly, 1 × 105 cells were seeded onto coverslips the day before the experiment. For infections, bacteria were harvested from agar plates and the OD600 was adjusted to 2.0 (∼1 × 109 bacteria per ml). Bacteria were used at a multiplicity of infection of 100 and sedimented onto cultured cells by centrifugation for 5 min at 300 × g at room temperature. Infections were stopped after 30 min by adding 3.75% PBS-buffered PFA. Bacterial adhesion was analyzed by CLSM.
Immunostaining and CLSM.
B. henselae was resuspended in PBS, air dried on glass slides, and fixed in 3.75% PBS-buffered PFA. Immunostaining was performed as described previously (29). Briefly, the bacteria were washed three times in PBS after fixation and each of the following incubation steps, and nonspecific binding was blocked by incubation with 0.2% bovine serum albumin for 15 min. The bacteria were incubated with a BadA-specific rabbit immunoglobulin G antibody (Ab) for 1 h, followed by incubation with a fluorescein isothiocyanate (FITC)-conjugated secondary anti-immunoglobulin G Ab (29).
For adhesion assays, HUVECs (1 × 105) were seeded onto coverslips and infected with B. henselae, and infection was stopped after 30 min by adding 3.75% PFA. Filamentous actin was stained with tetramethyl rhodamine isothiocyanate (TRITC)-labeled phalloidin for 30 min, and bacterial and host cell DNA was stained with DAPI for 10 min. FITC-conjugated secondary Ab and TRITC-labeled phalloidin were purchased from Dianova (Hamburg, Germany) and Sigma (Deisenhofen, Germany). Cellular fluorescence was evaluated using a Leica DM IRE 2 confocal laser scanning microscope. Three different fluorochromes were detected, representing the green (FITC), red (TRITC), and blue (DAPI) channels. Images were digitally processed with Photoshop 6.0 (Adobe Systems).
Western blotting and detection of Fn binding.
For Western blotting, B. henselae strains were harvested from agar plates after 5 days of cultivation, and the OD600 was adjusted to 2.0 (∼1.0 × 109 bacteria per ml) for normalization. Equal volumes of bacterial suspensions were centrifuged, and the resulting bacterial pellets (∼1.0 × 107 bacteria) were lysed in sodium dodecyl sulfate sample buffer, heated at 98°C for 3 min and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 12% gels. For immunoblotting, proteins were transferred onto nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). Membranes were blocked for 1 h in 5% skim milk powder in 25 mM Tris, pH 7.5, 0.15 M NaCl, and 0.05% Tween 20. For the detection of BadA, membranes were incubated with a BadA-specific rabbit Ab (29) overnight. To analyze the Fn-binding capacity, the membranes were incubated with a monoclonal anti-Fn Ab (Becton Dickinson). The blots were developed using horseradish peroxidase-conjugated secondary Abs, and signals were visualized via chemiluminescence (ECL; Amersham).
PCRs.
For PCR analysis of badA, bacterial genomic DNA was isolated using either the Genomic-tip 100/G kit (QIAGEN, Hilden, Germany) or the Bacterial Genomic DNA kit (Metabion, Martinsried, Germany) according to the manufacturer's instructions.
Long-distance PCRs of full-length badA were performed with the Expand Long Template PCR system (Roche, Mannheim, Germany) and the primers badA-LRf1 (5′-TTACATACCGGATCCCACTCAATATAAAGAAACACTCG-3′) and badA-LRr2 (5′-ACTGCATAAGGATCCGACGTGTTTCACCAGCTGC-3′). Primer badA-LRf1 binds 484 bp upstream of badA, and primer badA-LRr2 binds 182 bp downstream of badA. The cycling conditions were as follows: denaturation at 92°C for 10 s, annealing at 58°C for 10 s, and extension at 68°C for 8 min for 10 cycles, followed by 15 cycles with denaturation at 92°C for 10 s, annealing at 58°C for 30 s, and extension at 68°C for 8 min, plus 20 s of elongation for each successive cycle. Cycling was started with an initial 2-min denaturation at 92°C and a final 7-min extension at 68°C. Long-distance PCRs of the badA stalk region were performed using the primers badAf6 (5′-AAAGCATTAAGGGGAATGATATCAG-3′) and badAr7 (5′-CTCATACTTAACAGCACTATCTGC-3′). The cycling conditions were as follows: denaturation at 92°C for 10 s, annealing at 55°C for 30 s, and extension at 68°C for 6 min for 10 cycles, followed by 15 cycles with denaturation at 92°C for 10 s, annealing at 55°C for 30 s, and extension at 68°C for 6 min, plus 5 s of elongation for each successive cycle. Cycling was started with an initial 2-min denaturation at 92°C and a final 7-min extension at 68°C. PCR products were analyzed using 0.6% agarose gels.
All other PCRs were carried out according to standard protocols with Taq polymerase purchased from Metabion. For analysis of the putative promoter region of badA, primers badAPf1 (5′-TATTGGATCCTGAATTTACAGAGTGTAAGC-3′) and badAr9 (5′-TTACAGTTTCCAATGAGAGC-3′) were used. The primers for the amplification of the head region were badAf5 (5′-CCAATAATAAAACTGCATAATGATTCGACG-3′) and badAr8 (5′-TGATATCATGGATCCTTATGCTTTTAGCTGTGC-3′). PCRs of the membrane anchor region were performed with primers badAf4 (5′-GATAGTACTGGCAAGAAAACG-3′) and badAr5 (5′-CCAATAATAAAACTGCATAATGATTCGACG-3′). The PCR products were sequenced, and sequence alignments were made in Clustal W (6; http://www.ebi.ac.uk/clustalw/).
Nucleotide sequence accession numbers.
The promoter, head, and membrane anchor sequences of the various B. henselae strains (Table 1) are given under GenBank accession numbers DQ779056 (ATCC 49882), DQ779057 (ATCC 49882 variant), DQ779058 (ATCC 49793), DQ779059 (FR96BK3), DQ779060 (FR96BK38), DQ779061 (Berlin-1), DQ779062 (Berlin-2), and DQ779063 (G-5436). The complete badA sequence and the putative promoter sequence of B. henselae strain Marseille are available under GenBank accession numbers DQ665674 and DQ779055, respectively (generation of the complete badA sequence is not described in this report).
RESULTS
Analysis of BadA expression in various B. henselae strains.
BadA has been characterized as an important pathogenicity factor of B. henselae (29). Expression of BadA is lost after bacteria are passaged in vitro (4, 17), but unfortunately, in many studies the passage number of bacteria was not stated and expression of BadA was not tested. Therefore, we wanted to analyze different B. henselae strains for BadA expression. The strains we used were three cat isolates and five human isolates from different geographical regions (Table 1). The BadA-positive strain B. henselae Marseille (early passage) and the highly passaged BadA-negative strain B. henselae Marseille variant (originally described as B. henselae Pil−) (17, 29) were used as positive and negative controls, respectively.
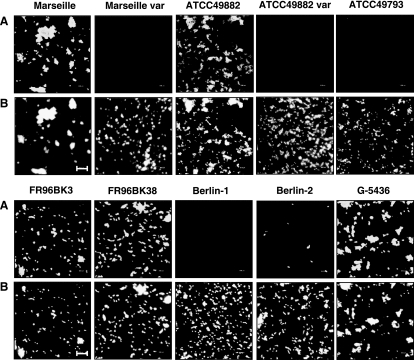
First, BadA expression was analyzed by immunofluorescence using BadA-specific Abs (Fig. 1). The results showed that B. henselae strains Marseille, ATCC 49882, Freiburg 96BK3 (FR96BK3), FR96BK38, and G-5436 expressed BadA; in contrast, B. henselae strains Marseille variant, ATCC 49882 variant, ATCC 49793, and Berlin-1 did not. In strain Berlin-2, the vast majority of bacteria were BadA negative and only some expressed BadA. These data show that expression of BadA differs between various B. henselae strains independently of their origin of isolation (human or cat) or geographic region (Europe or United States).
FIG. 1.
BadA expression in different B. henselae strains. (A) BadA was detected on the surfaces of the strains Marseille, ATCC 49882, FR96BK3, FR96BK38, and G-5436 by immunofluorescence using a specific anti-BadA-Ab (var., variant strain). Note that in strain Berlin-2, only a few bacteria are BadA positive. (B) For internal control, bacterial DNA was stained with DAPI. Scale bar, 8 μm.
Correlation between BadA expression and biological functions of different B. henselae strains.
It has been shown that BadA of B. henselae and VompA to -C of B. quintana are important for autoagglutination (4, 37). Moreover, expression of BadA is crucial for the adhesion of B. henselae to extracellular-matrix proteins (e.g., Fn) and to ECs (29). Therefore, we next analyzed the different B. henselae strains for (i) Fn binding, (ii) autoagglutination, and (iii) adhesion to ECs.
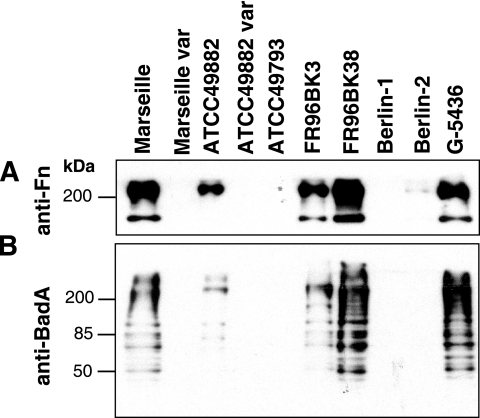
Bacterium-bound Fn was detected by Western blotting of whole bacterial lysates using anti-Fn Abs (Fig. 2). For control reasons and to confirm the data obtained by immunofluorescence (see above), expression of BadA was analyzed in parallel by Western blotting using anti-BadA Abs. The results clearly showed that only the BadA-positive strains (Marseille, ATCC 49882, FR96BK3, FR96BK38, and G-5436) bound Fn, whereas BadA-negative strains (Marseille variant, ATCC 49882 variant, ATCC 49793, and Berlin-1) did not. In accordance with immunofluorescence, strain Berlin-2 showed only very weak Fn binding.
FIG. 2.
Analysis of the Fn-binding capacity of B. henselae and detection of BadA expression by Western blotting. (A) Fn (240 kDa) bound to bacteria was detected in bacterial lysates using an anti-Fn Ab. Note that only BadA-positive strains bind Fn. Strain Berlin-2, in which only a few bacteria express BadA (see Fig. 1), shows very weak Fn binding. (B) For internal control, BadA (∼340 kDa) was detected in bacterial lysates by using a specific anti-BadA Ab. Multiple bands are interpreted as degradation products of the high-molecular-weight BadA (29).
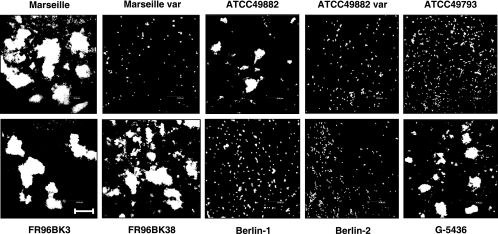
Autoagglutination of B. henselae was analyzed by CLSM after incubation of bacteria in suspension. Only BadA-positive strains (Marseille, ATCC 49882, FR96BK3, FR96BK38, and G-5436) autoagglutinated, whereas BadA-negative bacteria (Marseille variant, ATCC 49882 variant, ATCC 49793, and Berlin-1) were not self-adherent (Fig. 3).
FIG. 3.
BadA-dependent autoagglutination of different B. henselae strains. Bacteria were resuspended in PBS, incubated for 60 min, stained with DAPI, and analyzed by CLSM. Note that only BadA-positive strains show autoagglutination. Scale bar, 20 μm.
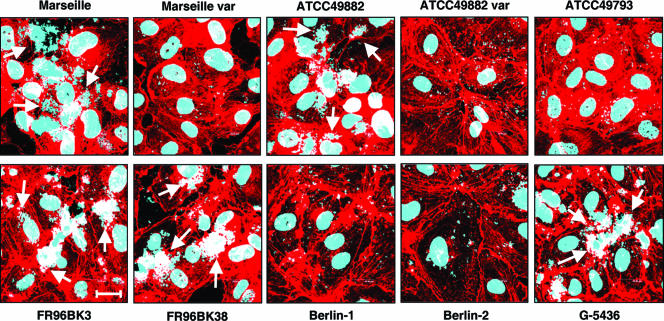
Moreover, we investigated the adherence of the different strains to ECs. For this purpose, HUVECs were infected with B. henselae (multiplicity of infection, 100), and the number of adherent bacteria was analyzed by CLSM 30 min after infection (Fig. 4). The BadA-negative strains (Marseille variant, ATCC 49882 variant, ATCC 49793, and Berlin-1) adhered significantly less to HUVECs than BadA-positive strains (Marseille, ATCC 49882, FR96BK3, FR96BK38, and G-5436). B. henselae Berlin-2 appeared in these functional assays like the BadA-negative strains, most likely because most of the bacteria were BadA negative (see above).
FIG. 4.
BadA-dependent adhesion of different B. henselae strains to endothelial cells. HUVECs were infected with B. henselae, and adhesion of the bacteria was analyzed after 30 min by CLSM. Bacteria and host cell nuclei were stained with DAPI (blue signal); filamentous actin was stained with TRITC-labeled phalloidin (red signal). Note that only BadA-positive strains adhere strongly to HUVECs (arrows). Scale bar, 20 μm.
Taken together, BadA expression of different B. henselae strains correlated perfectly with function (autoagglutination, Fn binding, and EC adherence). These results suggest that the proposed functions of BadA originally identified in the B. henselae Marseille strain (Fn binding and adherence to ECs) (29) or of the BadA-homologous VompA to -C in B. quintana (autoagglutination) (37) are valid for the whole genus Bartonella.
Genetic analysis of badA in different B. henselae strains.
Loss of BadA expression after B. henselae was passaged in vitro on agar plates has been described as phase variation (4, 17). However, the actual mechanism of gene regulation is not known. So far, the sequence of the genomic region harboring badA is available only for the special B. henselae ATCC 49882 substrain used in the genome-sequencing project (1) and for the Marseille (GenBank accession number DQ665674) and the BadA-negative Marseille variant strains (29).
The size of badA from the strain ATCC 49882, for which the genomic sequence has been published (1), is about 9.1 kb. It contains a 1-bp deletion at position 9054, leading to a premature stop codon upstream of the membrane anchor sequence (29). B. henselae Marseille contains a complete badA gene of 9.25 kb, while the corresponding BadA-negative variant strain contains an 8.5-kb deletion spanning the 5′ end and the promoter region of badA (29). As the reason for divergent BadA expression of the strains used in this study is not known, we analyzed the respective badA genes and the putative promoter regions in greater detail.
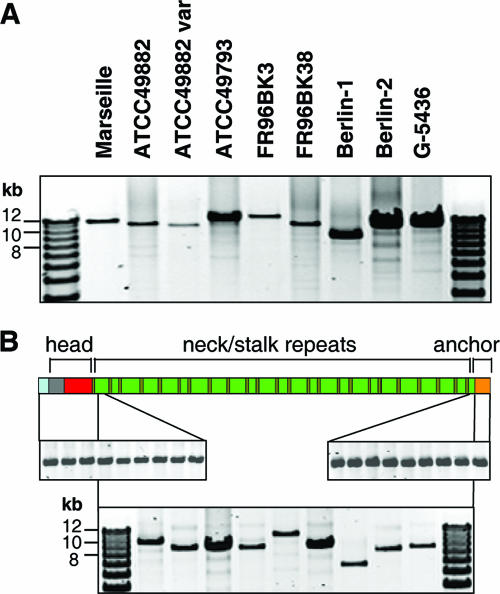
First, the presence of badA was confirmed by long-distance PCR (see Materials and Methods). The PCR revealed amplicons in all strains showing the presence of badA apart from the B. henselae Marseille variant (data not shown), in which the binding site of the upstream primer is missing (29). Interestingly, badA genes differ in size, ranging from about 9 to 12 kb (Fig. 5A). Amplification and sequencing of the nonrepetitive head and membrane anchor regions of badA revealed that they are exactly the same size in every strain investigated here (Fig. 5B). Therefore, we suggested that the differences in the size of badA are probably caused by variations in the lengths of the stalk sequences in various B. henselae strains. For this reason, we performed long-distance PCRs of the stalk region. In fact, we found the stalk region of badA to be highly variable in length (∼7 to 10 kb) (Fig. 5B). Notably, no deletions, such as are known from the B. henselae Marseille variant (deletion size, 8.5 kb), leading to a loss of essential parts of the gene or the promoter region (29) were detected, and moreover, it seems that there is no correlation between the size of the gene and BadA expression.
FIG. 5.
Presence and lengths of badA, badAhead, badAstalk, and badAmembrane anchor of different B. henselae strains analyzed by PCR. (A) Long-distance PCRs were performed (for details, see Materials and Methods), and the PCR products were analyzed in a 0.6% agarose gel. All B. henselae strains harbor badA, although there are differences in the size of the gene (∼9 to 12 kb). (B) Detection of the badAhead- (upper left), badAstalk- (lower middle) and badAmembrane anchor-coding sequences (upper right) in different B. henselae isolates. Note that differences in size are detectable only in badAstalk. For reasons of clarity, BadA is schematically depicted with the signal peptide (light blue), the head sequence (gray and red), 24 neck/stalk repeats (brown and green, respectively) and the membrane anchor (orange).
Analysis of the head domain.
Next, the head-coding sequences of badA were sequenced and analyzed for differences that could explain possible mechanisms of gene regulation. The data revealed 100% sequence homology of the different badA head regions (ending before the first neck sequence) either to that of B. henselae ATCC 49882 (the sequence reference strain) (1) or to that of B. henselae Marseille (GenBank accession number DQ665674). Although two clusters of badA head domain sequences were detected (cluster A, B. henselae Marseille, FR96BK3, and Berlin-2; cluster B, B. henselae ATCC 49882, ATCC 49882 variant, ATCC 49793, FR96BK38, and G-5436), these differences did not correlate with BadA expression (Fig. 6A). Surprisingly, strain Berlin-1, belonging to cluster B, exhibited a single-base deletion at position 585. The resulting frameshift introduced a stop codon within the head region, explaining the BadA-negative phenotype of the strain.
FIG. 6.
Comparison of the head and membrane anchor regions of BadA in different B. henselae strains. A Clustal W alignment of (A) amino acids 191 to 357 (part of the head) and (B) the C-terminal 130 amino acids (membrane anchor) is shown. Amino acids 1 to 190 are not shown, as they are identical in all strains. Differences in the sequences are marked in yellow and gray. ATCC49882seq, sequence of the published ATCC 49882 isolate (1). In this strain, a stop codon is introduced in the membrane anchor-coding sequence due to a 1-bp deletion. The strains cluster, in both the head and the membrane anchor regions, in two groups, with B. henselae Marseille as an intermediate between the groups. The four transmembrane β-strands of the membrane anchor are underlined.
Analysis of the membrane anchor domain.
Analysis of the membrane anchor region (starting after the last neck sequence) showed that none of the strains contains the same 1-bp deletion as that known from the particular ATCC 49882 type strain sequence (1, 29). Even the ATCC 49882 strain we used in this study contains the complete membrane anchor sequence without any frameshift mutations. Again, the strains cluster in two groups of identical sequences (cluster A, FR96BK3 and Berlin-2; cluster B, ATCC 49882, ATCC 49882 variant, ATCC 49793, Berlin-1, FR96BK38, and G-5436), with the B. henselae Marseille sequence as an intermediate between the clusters (Fig. 6B), and sequence differences do not correlate with BadA expression. As there are only minor amino acid exchanges between the clusters, we suggest that the membrane anchors of all strains are probably functional and should allow the transport of the passenger domain of BadA.
Analysis of the putative promoter region of badA.
For analysis of the putative promoter region of badA, ∼650 bp upstream of the start codon were sequenced (for an alignment of the sequences, see Fig. S1 in the supplemental material). Sequence comparison revealed only minor differences between the strains. The sequences cluster in two groups of identical sequences, as shown for the head and membrane anchor regions (cluster A, strains Marseille, FR96BK3, and Berlin-2; cluster B, ATCC 49882, ATCC 49882 variant, ATCC 49793, Berlin-1, and G-5436). The sequence of FR96BK38 is an intermediate between the groups and contains some unique bases. As both BadA-positive and BadA-negative strains are present in both clusters, it can be suggested that the differences in the promoter region do not correlate with the expression of BadA.
DISCUSSION
The TAA BadA (originally described as “pilus” [4]) is an important pathogenicity factor of B. henselae (29). Loss of BadA expression after passaging has been described as phase variation (4, 17, 20), but the actual mechanism of gene regulation is not known. In the current study, we investigated which strains of B. henselae express BadA on their surfaces and whether this expression correlates with function. Moreover, we tried to elucidate mechanisms involved in the regulation of BadA expression. For this purpose, BadA expression by different B. henselae strains isolated from humans and cats in different geographical regions was analyzed, revealing dramatic differences between and within distinct B. henselae strains.
Five of 10 B. henselae strains (Marseille, ATCC 49882, FR96BK3, FR96BK38, and G-5436) expressed BadA (Fig. 1 and 2), whereas four strains (Marseille variant, ATCC 49882 variant, ATCC 49793, and Berlin-1) did not. In one strain (B. henselae Berlin-2), only a few bacteria expressed BadA. Among the strains we investigated, no correlation between BadA expression and the source of the pathogen (human or cat isolates and different geographic regions of the first isolation) could be observed.
It was shown earlier that BadA is crucial for Fn binding and adherence to host cells (29). The VompA to -C proteins were shown to mediate autoagglutination and collagen binding (37), and these Vomp proteins were recently described as representing TAAs highly homologous to BadA (22). Therefore, we analyzed whether the expression of BadA on the various strains correlates with Fn binding, adherence to ECs, and autoagglutination. In fact, all BadA-expressing strains showed these biological characteristics, which were absent when BadA was not expressed. These results confirm the crucial role of BadA in the infection process that was described earlier for the particular B. henselae Marseille strain (29).
Phase variation is defined as switching the expression of a certain phenotype on and off. Usually, this happens randomly and at high frequency. One possible mechanism for phase variation is slipped-strand mispairing, leading to variations in the number of short sequence repeats located either in the promoter or within a gene, influencing gene expression (13). Such a mechanism of gene regulation was shown, for example, for the BadA-homologous Neisseria adhesin A (NadA) of Neisseria meningitidis: here, a TAAA repeat tract is located upstream of the putative −35 element of the nadA promoter (25). Variations in the number of these repeats were associated with changes in the level of nadA expression by altering the binding of the transcriptional regulator integration host factor to the nadA promoter (24). Similarly, expression of UspA1 of M. catarrhalis, another TAA highly homologous to BadA (22), exhibits phase variation, depending on the number of guanine residues in a homopolymeric poly(G) tract upstream of uspA1 (21). Based on these observations, we analyzed the putative promoter regions of badA in all B. henselae strains tested here for such sequence differences, which might explain a mechanism of phase variation. However, we found only minor single-nucleotide exchanges that did not correlate with BadA expression. Therefore, it can be assumed that (in contrast to NadA of N. meningitidis and UspA1 of M. catarrhalis) BadA expression is not regulated by genetic changes in the promoter region.
Next, we analyzed the 5′ end of badA (coding for the signal sequence and the head domain) for sequence repeats that might facilitate phase variation. We found that in B. henselae Berlin-1, the deletion of one guanine in a stretch of six guanine residues present in all other B. henselae strains (possibly caused by slipped-strand mispairing) leads to the introduction of a stop codon within the head-coding sequence (Fig. 6). However, as this mutation occurs in only one BadA-negative strain, this is probably not a common mechanism regulating BadA expression. Remarkably, in the sequenced B. henselae ATCC 49882 isolate, a similar phenomenon of a 1-bp deletion leads to a frameshift and to the introduction of a stop codon upstream of the membrane anchor-coding sequence (1, 29). Again, none of the strains we have tested contained this deletion. All other differences we found in the head and membrane anchor sequences resulted in only minor changes in the deduced amino acid sequences that neither correlate with BadA expression nor should affect the structure or function of BadA. Based on these results, it might be speculated that single-base deletions frequently affect BadA expression. Because of the enormous size of the badA gene (∼9 to 12 kb) (Fig. 5A), mutations (including single-base deletions) might become more likely for stochastic reasons.
Additionally, it can be suggested that recombination events lead to a loss of TAA expression. For instance, the deletion of an 8.5-kb fragment spanning from the 5′-terminal end of the open reading frame upstream of badA (BH01490) to the 5′-terminal end of badA causes the lack of BadA expression in the B. henselae Marseille variant (29). A similar observation was made for B. quintana, in which a deletion of two vomps (vompA and -B) within the vomp gene cluster (vompA to -D) was detected (37). It is worth mentioning that we generated a second BadA-negative variant strain of B. henselae Marseille after 55 passages. In this strain, we could not detect a large deletion, as is known for B. henselae Marseille variant (see above). Here, the insertion of one guanine residue at position 4513 led to a stop codon within the stalk region, which is most likely responsible for the lack of BadA expression as shown by immunofluorescence and immunoblotting (data not shown). From all of these data, it can be suggested that within one strain at least two different events (e.g., recombination or single-base deletion or insertion) might cause the loss of BadA expression.
The observed variations in the total size of badA are due to variations in the length of the repetitive neck/stalk sequences; the sizes of the head and membrane anchor sequences turned out to be equal in all B. henselae strains investigated here (Fig. 5B). The highly repetitive nature of the neck/stalk fragments should facilitate recombination events, resulting in an expansion or contraction of their copy numbers (22). This suggestion is supported by the analysis of the vomp genes of B. quintana, which are constructed very similarly to badA but differ mainly in the number of their neck/stalk repeats (BadA, 24 neck repeats; VompA to -C, 6 neck repeats; VompD, 7 neck repeats) (32). It can be assumed that these recombinations lead to frameshifts in the neck/stalk repeats affecting BadA expression (22) (Fig. 5). However, because of the enormous length (∼7 to 10 kb) and the highly repetitive nature of the badA neck/stalk repeats, we were unfortunately not able to determine the sequences of these parts of the gene in strains other than B. henselae Marseille and its variants. Why the number of TAA neck/stalk repeats within the genus Bartonella differ by up to four times and whether this has any biological impact on the infection process are unclear and need to be elucidated further.
It might be speculated that expression of BadA is crucial for the virulence of B. henselae when infecting humans or the animal reservoir host. Because of its enormous size, expression of BadA should be a highly energy-consuming process, limiting the growth of freshly isolated and BadA-expressing wild-type strains. Therefore, loss of BadA expression upon extensive passaging on agar plates in vitro without any ecological pressure might improve the growth of BadA-negative variants. This is supported by the observation that BadA (“pilus”)-negative strains grow much faster on agar plates than wild-type bacteria (4, 18, 28). It can be speculated that, once a mutation affecting BadA expression occurs, BadA-negative strains overgrow the slowly growing BadA-positive wild-type colonies. This observation is supported by the presence of only a few BadA-positive bacteria in the culture of B. henselae Berlin-2 (Fig. 1) and the observation that the percentage of BadA-positive B. henselae Marseille decreases gradually with passage number (data not shown). According to this, further passaging of B. henselae Berlin-2 should result in a completely BadA-negative strain.
It is important to note that work on the pathogenicity of B. henselae was often performed using bacteria with variable, unstated (7, 23, 27, 31), and sometimes high passage numbers (9, 10) that were not tested for BadA expression. Because several B. henselae substrains (e.g., the ATCC 49882 variant) do not express BadA, we strongly emphasize that BadA expression should be evaluated first when performing infection experiments with B. henselae, as BadA determines host cell interaction (15, 29). This observation is also important in terms of interpreting serological results obtained with B. henselae antigen (5), as BadA has been shown to be an immunodominant surface protein of B. henselae (29).
Taken together, our results represent the first systematic approach analyzing BadA expression in different B. henselae strains. Our data suggest that different events (single-base deletions or insertions and recombinations) affect BadA expression, resulting in the coexistence of BadA-positive and BadA-negative substrains within one B. henselae type strain (e.g., B. henselae ATCC 49882). Further analysis (e.g., functional promoter analysis) is needed to understand the mechanisms regulating the expression of this important pathogenicity factor of B. henselae.
Supplementary Material
Acknowledgments
We thank Ingo B. Autenrieth for continuous support, Stephan Schuster and Christa Lanz for helping to sequence B. henselae Marseille badA, Andrei Lupas for critical discussion, and Diana Neumann for excellent technical assistance.
This work was supported by grants from the Deutsche Forschungsgemeinschaft to V.A.J.K. and from the University of Tübingen (Center for Interdisciplinary Clinical Research, junior group program).
Editor: J. L. Flynn
Footnotes
Published ahead of print on 23 October 2006.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alsmark, C. M., A. C. Frank, E. O. Karlberg, B. A. Legault, D. H. Ardell, B. Canback, A. S. Eriksson, A. K. Naslund, S. A. Handley, M. Huvet, B. LaScola, M. Holmberg, and S. G. Andersson. 2004. The louse-borne human pathogen Bartonella quintana is a genomic derivative of the zoonotic agent Bartonella henselae. Proc. Natl. Acad. Sci. USA 101:9716-9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, B. E., and M. A. Neuman. 1997. Bartonella spp. as emerging human pathogens. Clin. Microbiol. Rev. 10:203-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvand, M., C. Wendt, T. Regnath, R. Ullrich, and H. Hahn. 1998. Characterization of Bartonella henselae isolated from bacillary angiomatosis lesions in a human immunodeficiency virus-infected patient in Germany. Clin. Infect. Dis. 26:1296-1299. [DOI] [PubMed] [Google Scholar]
- 4.Batterman, H. J., J. A. Peek, J. S. Loutit, S. Falkow, and L. S. Tompkins. 1995. Bartonella henselae and Bartonella quintana adherence to and entry into cultured human epithelial cells. Infect. Immun. 63:4553-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1999. Serodiagnosis of emerging infectious diseases: Bartonella and Ehrlichia infections. Centers for Disease Control and Prevention, Manassas, VA.
- 6.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31:3497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabo, S. M., A. W. Confer, B. E. Anderson, and S. Gupta. 2006. Bartonella henselae Pap31, an extracellular matrix adhesin, binds the fibronectin repeat III13 module. Infect. Immun. 74:2513-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dehio, C. 2005. Bartonella-host-cell interactions and vascular tumour formation. Nat. Rev. Microbiol. 3:621-631. [DOI] [PubMed] [Google Scholar]
- 9.Dehio, C., M. Meyer, J. Berger, H. Schwarz, and C. Lanz. 1997. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J. Cell Sci. 110:2141-2154. [DOI] [PubMed] [Google Scholar]
- 10.Dehio, M., M. Quebatte, S. Foser, and U. Certa. 2005. The transcriptional response of human endothelial cells to infection with Bartonella henselae is dominated by genes controlling innate immune responses, cell cycle, and vascular remodelling. Thromb. Haemost. 94:347-361. [DOI] [PubMed] [Google Scholar]
- 11.Drancourt, M., R. Birtles, G. Chaumentin, F. Vandenesch, J. Etienne, and D. Raoult. 1996. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet 347:441-443. [DOI] [PubMed] [Google Scholar]
- 12.Gilmore, R. D., Jr., T. M. Bellville, S. L. Sviat, and M. Frace. 2005. The Bartonella vinsonii subsp. arupensis immunodominant surface antigen BrpA gene, encoding a 382-kilodalton protein composed of repetitive sequences, is a member of a multigene family conserved among Bartonella species. Infect. Immun. 73:3128-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson, I. R., P. Owen, and J. P. Nataro. 1999. Molecular switches—the ON and OFF of bacterial phase variation. Mol. Microbiol. 33:919-932. [DOI] [PubMed] [Google Scholar]
- 14.Hoiczyk, E., A. Roggenkamp, M. Reichenbecher, A. Lupas, and J. Heesemann. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 19:5989-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kempf, V. A., M. Lebiedziejewski, K. Alitalo, J. H. Wälzlein, U. Ehehalt, J. Fiebig, S. Huber, B. Schütt, C. A. Sander, S. Müller, G. Grassl, A. S. Yazdi, B. Brehm, and I. B. Autenrieth. 2005. Activation of hypoxia-inducible factor-1 in bacillary angiomatosis: evidence for a role of hypoxia-inducible factor-1 in bacterial infections. Circulation 111:1054-1062. [DOI] [PubMed] [Google Scholar]
- 16.Kempf, V. A., M. Schaller, S. Behrendt, B. Volkmann, M. Aepfelbacher, I. Cakman, and I. B. Autenrieth. 2000. Interaction of Bartonella henselae with endothelial cells results in rapid bacterial rRNA synthesis and replication. Cell Microbiol. 2:431-441. [DOI] [PubMed] [Google Scholar]
- 17.Kempf, V. A., B. Volkmann, M. Schaller, C. A. Sander, K. Alitalo, T. Riess, and I. B. Autenrieth. 2001. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell Microbiol. 3:623-632. [DOI] [PubMed] [Google Scholar]
- 18.Koehler, J. E., F. D. Quinn, T. G. Berger, P. E. LeBoit, and J. W. Tappero. 1992. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N. Engl. J. Med. 327:1625-1631. [DOI] [PubMed] [Google Scholar]
- 19.Koretke, K. K., P. Szczesny, M. Gruber, and A. N. Lupas. 2006. Model structure of the prototypical non-fimbrial adhesin YadA of Yersinia enterocolitica. J. Struct. Biol. 155:154-161. [DOI] [PubMed] [Google Scholar]
- 20.Kyme, P., B. Dillon, and J. Iredell. 2003. Phase variation in Bartonella henselae. Microbiology 149:621-629. [DOI] [PubMed] [Google Scholar]
- 21.Lafontaine, E. R., N. J. Wagner, and E. J. Hansen. 2001. Expression of the Moraxella catarrhalis UspA1 protein undergoes phase variation and is regulated at the transcriptional level. J. Bacteriol. 183:1540-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linke, D., T. Riess, I. B. Autenrieth, A. Lupas, and V. A. Kempf. 2006. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol. 14:264-270. [DOI] [PubMed] [Google Scholar]
- 23.Maeno, N., H. Oda, K. Yoshiie, M. R. Wahid, T. Fujimura, and S. Matayoshi. 1999. Live Bartonella henselae enhances endothelial cell proliferation without direct contact. Microb. Pathog. 27:419-427. [DOI] [PubMed] [Google Scholar]
- 24.Martin, P., K. Makepeace, S. A. Hill, D. W. Hood, and E. R. Moxon. 2005. Microsatellite instability regulates transcription factor binding and gene expression. Proc. Natl. Acad. Sci. USA 102:3800-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin, P., T. van de Ven, N. Mouchel, A. C. Jeffries, D. W. Hood, and E. R. Moxon. 2003. Experimentally revised repertoire of putative contingency loci in Neisseria meningitidis strain MC58: evidence for a novel mechanism of phase variation. Mol. Microbiol. 50:245-257. [DOI] [PubMed] [Google Scholar]
- 26.Meng, G., N. K. Surana, J. W. St. Geme III, and G. Waksman. 2006. Structure of the outer membrane translocator domain of the Haemophilus influenzae Hia trimeric autotransporter. EMBO J. 25:2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musso, T., R. Badolato, D. Ravarino, S. Stornello, P. Panzanelli, C. Merlino, D. Savoia, R. Cavallo, A. N. Ponzi, and M. Zucca. 2001. Interaction of Bartonella henselae with the murine macrophage cell line J774: infection and proinflammatory response. Infect. Immun. 69:5974-5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regnery, R. L., B. E. Anderson, J. E. Clarridge, B. M. Rodriguez, D. C. Jones, and J. H. Carr. 1992. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J. Clin. Microbiol. 30:265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riess, T., S. G. Andersson, A. Lupas, M. Schaller, A. Schäfer, P. Kyme, J. Martin, J. H. Wälzlein, U. Ehehalt, H. Lindroos, M. Schirle, A. Nordheim, I. B. Autenrieth, and V. A. Kempf. 2004. Bartonella adhesin A mediates a proangiogenic host cell response. J. Exp. Med. 200:1267-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sander, A., C. Buhler, K. Pelz, E. von Cramm, and W. Bredt. 1997. Detection and identification of two Bartonella henselae variants in domestic cats in Germany. J. Clin. Microbiol. 35:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmiederer, M., R. Arcenas, R. Widen, N. Valkov, and B. Anderson. 2001. Intracellular induction of the Bartonella henselae virB operon by human endothelial cells. Infect. Immun. 69:6495-6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulte, B., D. Linke, S. Klumpp, M. Schaller, T. Riess, I. B. Autenrieth, and V. A. Kempf. 2006. Bartonella quintana variably expressed outer membrane proteins mediate vascular endothelial growth factor secretion but not host cell adherence. Infect. Immun. 74:5003-5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welch, D. F., D. A. Pickett, L. N. Slater, A. G. Steigerwalt, and D. J. Brenner. 1992. Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J. Clin. Microbiol. 30:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wollmann, P., K. Zeth, A. Lupas, and D. Linke. 2006. Purification of the YadA membrane anchor for secondary structure analysis and crystallization. Int. J. Biol. Macromol. 15:3-9. [DOI] [PubMed] [Google Scholar]
- 35.Zbinden, R., M. Hochli, and D. Nadal. 1995. Intracellular location of Bartonella henselae cocultivated with Vero cells and used for an indirect fluorescent-antibody test. Clin. Diagn. Lab. Immunol. 2:693-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zbinden, R., N. Michael, M. Sekulovski, A. von Graevenitz, and D. Nadal. 1997. Evaluation of commercial slides for detection of immunoglobulin G against Bartonella henselae by indirect immunofluorescence. Eur. J. Clin. Microbiol. Infect. Dis. 16:648-652. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, P., B. B. Chomel, M. K. Schau, J. S. Goo, S. Droz, K. L. Kelminson, S. S. George, N. W. Lerche, and J. E. Koehler. 2004. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc. Natl. Acad. Sci. USA 101:13630-13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.