Abstract
Leishmaniasis affects 12 million people, but there are no vaccines in routine clinical use. Th1 polarizing vaccines that elicit long-term protection are required to prevent disease in susceptible populations. We recently showed that heterologous priming-boosting with tryparedoxin peroxidase (TRYP) DNA followed by TRYP-modified vaccinia virus Ankara (TRYP MVA) protected susceptible BALB/c mice from Leishmania major. Here we compared treatment with TRYP DNA with treatment with TRYP DNA/TRYP MVA. We found that equivalent levels of protection during the postvaccination effector phase correlated with equivalent levels of serum immunoglobulin G2a and gamma interferon (IFN-γ) in draining lymph nodes. In contrast, challenge infection during the memory phase revealed that there was enhanced clinical efficacy with TRYP DNA/TRYP MVA. This correlated with higher levels of effector phase splenic IFN-γ, sustained prechallenge levels of memory phase IFN-γ, and a more polarized post-L. major challenge Th1 response compared to the Th2/Treg response. Thus, TRYP DNA/TRYP MVA, but not TRYP DNA alone, provides long-term protection against murine leishmaniasis.
Leishmaniasis is caused by intracellular protozoan parasites transmitted by the bite of a sand fly, and the diverse clinical manifestations range from localized cutaneous lesions to fatal visceral infection. The disease prevalence is estimated to be 12 million people, and there are 1.5 million new cases annually. There are no vaccines in routine use. Experimental infections of inbred mice with Leishmania major defined the Th1/Th2 paradigm (for reviews, see references 16 and 26) and demonstrated that primary immunity to L. major in resistant mice requires the development of a polarized Th1 response (11, 31, 32). In contrast, susceptibility in BALB/c mice was associated with an aberrant Th2 response resulting from the early production of interleukin-4 (IL-4) by a restricted population of Vβ4Vα8 CD4+ T cells (12, 13). These studies supported the hypothesis that immunotherapy shifting the balance from IL-4 to gamma interferon (IFN-γ) would provide the key to vaccine success. The challenge for developing a vaccine against Leishmania spp., like the challenge for developing vaccines against other intracellular pathogens, such as Mycobacterium tuberculosis, is thought to be induction and maintenance of a cell-mediated immune response that produces IFN-γ to activate macrophages to kill the pathogen.
The vaccination strategies employed to induce protective immunity in experimental models of leishmaniasis have included vaccination with recombinant Leishmania antigens, such as a Leishmania homologue of the receptor for activated C kinase (LACK) plus IL-12 as an adjuvant, vaccination with live attenuated parasites, vaccination with plasmid DNA encoding single or multiple parasite antigens, and vaccination with live recombinant vectors, such as Salmonella spp., Mycobacterium bovis BCG, or vaccinia virus (for a review, see reference 19). While all of these studies have resulted in some degree of efficacy, long-lived protection has rarely been observed.
We recently showed that heterologous priming-boosting with DNA followed by modified vaccinia virus Ankara (MVA) expressing the Leishmania antigen tryparedoxin peroxidase (TRYP), alternatively referred to as thiol-specific antioxidant (3), protected susceptible BALB/c mice from cutaneous leishmaniasis (34). Here, we compared TRYP delivered as DNA alone with heterologous priming-boosting with TRYP DNA followed by TRYP MVA. We found that while equivalent protection was induced if mice were challenged during the effector phase of the response to vaccination, only heterologous priming-boosting with TRYP DNA followed by TRYP MVA induced long-term protection. This correlated with higher levels of effector phase splenic IFN-γ, which may have reflected induction of a central memory response, sustained prechallenge memory phase IFN-γ, and a post- L. major challenge Th1 response that was more polarized than the Th2 response.
MATERIALS AND METHODS
Mice.
Female 5- to 6-week-old BALB/c mice were purchased from Charles River Laboratories (Margate, United Kingdom) and were maintained at Central Biomedical Services (University of Cambridge, United Kingdom) under pathogen-free conditions. All procedures were carried out under United Kingdom Government Home Office guidelines.
Plasmid construction and purification.
TRYP was amplified from cDNA clone lmf30 (accession number T67356), obtained from an L. major substrain LV39 (MRHO/SU/59/P) cDNA library (14), and was inserted downstream of the cytomegalovirus promoter into a modified version (without the neomycin resistance gene) of pcDNA3 (Invitrogen). Empty pcDNA3 was used as vector control. Plasmid DNA was purified using Endofree plasmid Maxi kits (QIAGEN Ltd., Crawley, United Kingdom) with pyrogen-free material, and the final pellet was resuspended in pyrogen-free phosphate-buffered saline.
Construction and purification of recombinant MVA.
Recombinant MVA was constructed as previously described (4, 34). For vaccinations, semipurified stocks of recombinant MVA grown in RK13 cells were prepared by ultracentrifugation through a sucrose cushion, resuspended in 10 mM Tris-HCl (pH 9), and stored at −80°C until they were needed. Expression of protein from MVA-infected culture lysate was demonstrated by Western blotting using pooled immune sera from TRYP DNA-vaccinated mice. The expected protein band at ∼22 kDa was observed (data not shown).
Preparation of crude and recombinant antigens.
Crude freeze-thawed parasite antigen (FTP) was prepared from stationary-phase promastigotes by resuspension in 10 mM Tris-HCl (pH 8.5), 0.5 M NaCl, 1 mM phenylmethylsulfonyl fluoride, 50 μg/ml leupeptin and three cycles of freezing and thawing over liquid nitrogen. Recombinant protein was prepared by cloning TRYP into the expression vector pET-15b (Novagen, Madison, WI) and transformation into Escherichia coli BL21(DE3) host cells (15). Recombinant proteins were purified by affinity chromatography after incubation of cleared supernatants with Ni-nitrilotriacetic acid agarose (QIAGEN). Proteins were eluted with 10 mM Tris-HCl (pH 8.5), 0.5 M NaCl, 200 mM imidazole, dialyzed, and purified further using Detoxi-Gel affinitypak columns (Perbio Science, Tattenhall, United Kingdom) to remove endotoxin. Protein contents were estimated using the Bio-Rad protein assay (Bio-Rad Laboratories, Hemel Hempstead, United Kingdom).
Immunization.
Groups of 14 mice were inoculated subcutaneously in the shaved rump with two 100-μg doses of TRYP or vector DNA 3 weeks apart. We designated these two doses of DNA the priming vaccination. After 5 weeks, mice were boosted intravenously with 1 × 106 PFU TRYP (referred to below as TRYP/TRYP) or vector MVA (referred to below as TRYP/Vec), which we designated the booster vaccination. Control mice received vector DNA followed by vector MVA (referred to below as Vec/Vec).
Infectious challenge.
L. major substrain LV39 promastigotes were cultured at 26°C in Schneider's insect medium (Sigma) supplemented with 10% fetal calf serum (Invitrogen), 2 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. BALB/c mice were challenged 2 weeks after the boost with 2 × 106 stationary-phase (days 5 to 6) promastigotes in the hind footpad. Footpad depth was determined by weekly measurement with vernier calipers.
In vivo recall.
Two weeks after the booster vaccination, BALB/c mice were inoculated in both hind footpads with 5 μg of FTP; 48 h later, mice were sacrificed and bled to obtain serum, and draining lymph nodes (LN) and spleens were removed.
IgG ELISA.
Sera for antibody testing were collected from vaccinated mice 48 h after the in vivo recall with FTP. Antigen-specific antibody subclasses were measured by an enzyme-linked immunosorbent assay (ELISA) using recombinant TRYP-coated plates and biotinylated rabbit anti-mouse immunoglobulin G1 (IgG1)- or IgG2a (Zymed Laboratories Inc., San Francisco, CA)-detecting antibodies. Total IgG was probed using biotinylated rabbit anti-mouse IgG (Dako). Detection was performed with streptavidin-horseradish peroxidase and the o-phenylenediamine substrate. Plates were read at 492 nm (OPTI-MAX; Molecular Devices).
Cytokine assays.
Pooled cells from spleens or draining (popliteal) LN were cultured in RPMI 1640 (Invitrogen) at a concentration of 6 × 105 cells/well in U-bottom 96-well plates and were stimulated for 72 h at 37°C in the presence of 5% CO2 with or without 10 μg/ml TRYP recombinant protein or 10 μg/ml FTP. Bone marrow-derived dendritic cells (DC) were obtained by in vitro stimulation for 5 to 7 days with 10 to 20 ng/ml granulocyte-macrophage colony-stimulating factor (Peprotech, London, United Kingdom) prior to overnight infection with opsonized stationary-phase L. major promastigotes (five parasites per DC). DC were washed twice and incubated at a concentration of 1 × 105 infected or uninfected DC per 6 × 105 splenocytes or LN cells for 72 h prior to the removal of cell supernatants. For blocking antibody experiments, cells were incubated with 10 μg/ml inhibitory CD4 (H129.19; NA/LE; Pharmingen) or CD8 (53-6.7; NA/LE; Pharmingen) monoclonal antibody or an isotype control (R35-95; NA/LE; Pharmingen). Supernatants were removed, and IL-4, IL-10, and IFN-γ levels were determined by a sandwich ELISA using antibodies obtained from Pharmingen.
Statistical analysis.
Statistical differences (P < 0.05) between the immunization groups were determined using the unpaired, two-tailed Student t test. The data below are representative of at least two replicate experiments.
RESULTS
TRYP/Vec DNA alone elicits protection equivalent to that provided by TRYP/TRYP DNA/MVA heterologous priming-boosting when mice are challenged with L. major 2 weeks after booster vaccination.
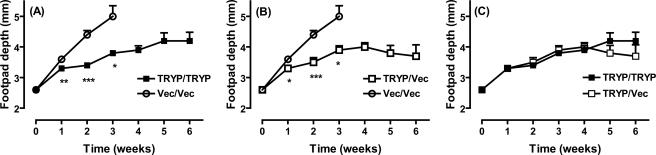
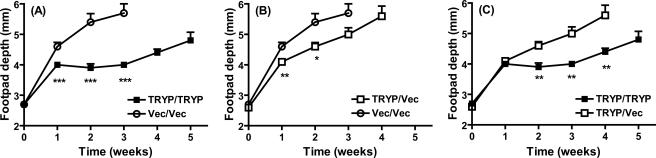
BALB/c mice were primed with two 100-μg doses of TRYP or vector DNA and boosted with 106 PFU MVA either expressing TRYP (TRYP/TRYP) or comprising vector only (TRYP/Vec or Vec/Vec), and they were challenged 2 weeks later (i.e., in the effector phase of the postvaccination immune response) with 2 × 106 promastigotes of L. major in the hind footpad (Fig. 1). TRYP DNA-vaccinated mice challenged in the effector phase were significantly protected compared to mice vaccinated with vector DNA alone (Fig. 1A and B). Boosting with TRYP MVA did not significantly enhance the protection provided by TRYP DNA alone, as demonstrated by footpad depth (Fig. 1C) and mouse survival rates (data not shown).
FIG. 1.
Clinical outcome following L. major challenge of BALB/c mice vaccinated with TRYP DNA/TRYP MVA (TRYP/TRYP), TRYP DNA/vector MVA (TRYP/Vec), or vector DNA/vector MVA (Vec/Vec) at 2 weeks after booster vaccination. Mice were vaccinated with two 100-μg doses of TRYP or vector DNA given 3 weeks apart and were boosted with 1 × 106 PFU of TRYP or vector MVA 2 weeks later. Mice were challenged 2 weeks after administration of the booster with 2 × 106 L. major promastigotes in the hind footpad, and footpad depth was monitored weekly with vernier calipers. The results are means ± standard errors for groups of mice in one experiment, which was representative of replicate experiments in which similar results were obtained. The asterisks indicate significant differences from the results for Vec/Vec-vaccinated mice, as determined by Student's t test (one asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001).
Specific IgG2a and IgG1 responses are equivalent in the effector phase for TRYP/Vec DNA and TRYP/TRYP heterologous DNA/MVA vaccination.
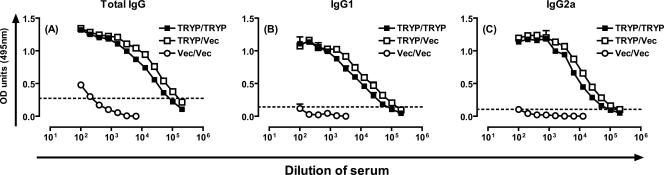
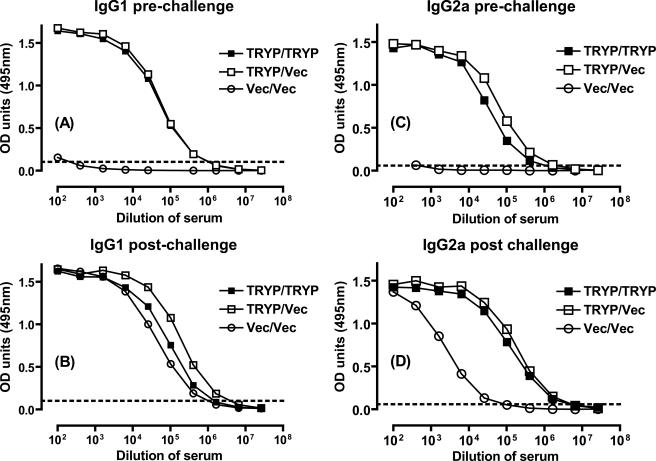
Two weeks after the booster vaccination, mice were inoculated in the footpad with FTP to elicit a recall response, and draining LN cells, splenocytes, and serum were removed 48 h later. Figures 2A to C show that the levels of TRYP-specific total immunoglobulin, IgG1, and IgG2a were almost equivalent in mice that received TRYP/TRYP and mice that received TRYP/Vec, demonstrating that TRYP DNA elicits a potent humoral immune response which is minimally boosted by TRYP MVA. Furthermore, the ratios of Th1 to Th2 were similar, with the ratio of IgG2a to IgG1 slightly more biased toward Th1 for TRYP/Vec-vaccinated mice (0.74; 0.63 in a repeat experiment) than for TRYP/TRYP-vaccinated mice (0.53; 0.52 in a repeat experiment). Thus, the humoral immune response reflected the clinical challenge shown in Fig. 1.
FIG. 2.
TRYP-specific IgG responses in mice vaccinated with TRYP/TRYP, TRYP/Vec, or Vec/Vec. Mice were vaccinated with two 100-μg doses of TRYP or vector DNA given 3 weeks apart and boosted with 1 × 106 PFU of TRYP or vector MVA 2 weeks later. At 2 weeks after administration of the booster, immune cells were recalled by in vivo inoculation of 5 μg FTP in the hind footpad, and serum, spleens, and draining LN were removed after 48 h. TRYP-specific total IgG (A), IgG1 (B), and IgG2a (C) were analyzed by ELISA using pooled sera. The antibody levels are the averages for duplicate absorbance (OD) determinations obtained using 12 serial twofold dilutions. The dotted line indicates the value used to determine end point titers. The results are representative of the results of replicate experiments in which similar results were obtained.
Effector phase splenocyte, but not draining LN, IFN-γ levels are enhanced after boosting with TRYP MVA.
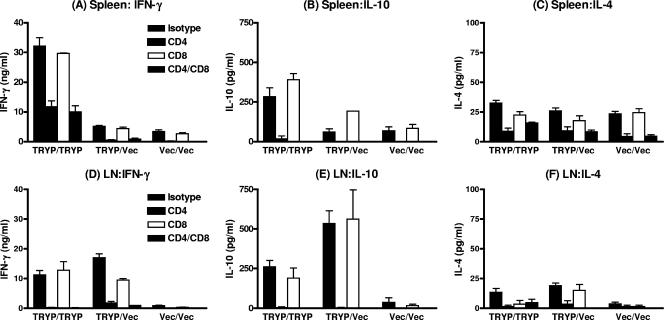
In vivo FTP-recalled draining LN cells removed from TRYP DNA-vaccinated mice released similar levels of IFN-γ upon restimulation in vitro with recombinant TRYP protein, regardless of whether mice were boosted with TRYP or vector MVA (Fig. 3D). This result mirrors the immunoglobulin responses (Fig. 2) and clinical phenotype (Fig. 1) described above. In contrast, enhanced IFN-γ release (P < 0.001) was observed in splenocytes (Fig. 3A) derived from TRYP/TRYP-vaccinated mice compared to splenocytes derived from TRYP/Vec-vaccinated mice. The TRYP/Vec responses were only marginally higher than the Vec/Vec splenic IFN-γ levels (P < 0.05). Hence, TRYP MVA boosts IFN-γ release in the spleen but not in the draining LN and/or these cells failed to traffic to draining LN upon in vivo recall. In addition, in the TRYP/TRYP treatment group, the splenocyte IFN-γ levels were significantly higher than the levels in the corresponding draining LN (P < 0.001) (compare Fig. 1A and D), whereas for TRYP/Vec the opposite was true. For both splenocytes (Fig. 3A) and LN cells (Fig. 3D), antigen-specific IFN-γ was produced predominantly by CD4+ T cells rather than by CD8+ T cells.
FIG. 3.
TRYP-specific IFN-γ, IL-10, and IL-4 levels in BALB/c mice immunized with TRYP/TRYP, TRYP/Vec, or Vec/Vec. Two weeks after booster vaccination, in vivo recall was performed by footpad injection of 5 μg FTP 48 h prior to removal of splenocytes (A to C) and draining LN cells (D to F). Triplicate wells containing pooled LN cells or splenocytes were restimulated in vitro with 10 μg/ml TRYP for 72 h in the presence or absence of 10 μg/ml anti-CD4 or anti-CD8 monoclonal antibody, the supernatants were removed, and the IFN-γ (A and D), IL-10 (B and E), and IL-4 (C and F) levels were determined by ELISA. The results (means ± standard errors) are representative of the results of replicate experiments in which similar results were obtained.
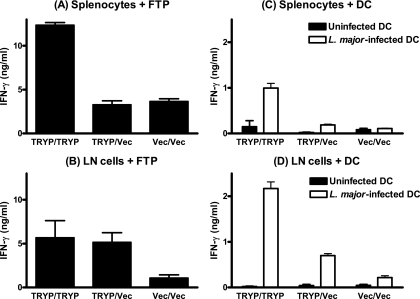
In vivo recalled splenocytes and LN cells were also restimulated in vitro either with FTP (Fig. 4A and B) or, to mimic more faithfully the in vivo response to parasite challenge, with L. major-infected DC (Fig. 4C and D). As observed after restimulation with TRYP protein, splenocytes released much higher levels of IFN-γ following vaccination with TRYP/TRYP than following vaccination with TRYP/Vec, although the magnitude of the response was markedly lower for DC stimulation. In draining LN, the FTP responses were equivalent for TRYP/TRYP and TRYP/Vec (Fig. 4B), whereas DC-stimulated LN cells from TRYP/TRYP-vaccinated mice released significantly higher levels of IFN-γ (P < 0.01) (Fig. 4D). These data suggest that effector cells from TRYP/TRYP-vaccinated mice may be more responsive to live L. major parasites than effector cells obtained from mice immunized with TRYP DNA alone, a phenomenon not observed when parasites are killed by freezing and thawing.
FIG. 4.
FTP- or L. major parasite-mediated IFN-γ responses in splenocyte or LN cells derived from BALB/c mice immunized with TRYP/TRYP, TRYP/Vec, or Vec/Vec. Two weeks after booster vaccination, in vivo recall was performed by footpad injection of 5 μg FTP 48 h prior to removal of splenocytes (A and C) and draining LN cells (B and D). Triplicate wells of pooled LN cells or splenocytes were restimulated in vitro with 10 μg/ml FTP or L. major-infected dendritic cells for 72 h, and supernatants were removed for detection of IFN-γ by ELISA. The results (means ± standard errors) are representative of the results of replicate experiments in which similar results were obtained.
Effector phase CD4-derived IL-10 responses in splenocytes and LN cells from TRYP/TRYP-vaccinated and TRYP/Vec-vaccinated mice.
As observed for IFN-γ, the CD4-derived IL-10 responses were significantly enhanced in spleens (P < 0.01) (Fig. 3B) but not in the draining LN (Fig. 3E) when heterologous primed-boosted TRYP/TRYP mice were compared to TRYP/Vec mice vaccinated with DNA alone. Indeed, the level of IL-10 released from splenocytes (Fig. 3B) derived from TRYP/Vec-vaccinated mice was not significantly higher than the level of IL-10 released from splenocytes derived from Vec/Vec-vaccinated mice, whereas the draining LN IL-10 responses (Fig. 3E) were greatest in the TRYP/Vec group. The IL-4 responses were also determined for both splenocytes (Fig. 3C) and draining LN cells (Fig. 3F). The responses were generally low (<50 pg/ml) and were not significantly greater in splenocytes from TRYP/TRYP-vaccinated or TRYP/Vec-vaccinated mice than in splenocytes from Vec/Vec-vaccinated mice. In draining LN, the IL-4 responses in TRYP/TRYP- and TRYP/Vec-vaccinated mice were equivalent. In our previous work (34) we showed that the ratio IFN-γ to IL-10, which can reflect Th1/Th2 activity as well as Th1/Treg activity, was a good predictor of vaccine outcome. In contrast to the Th1/Th2 bias indicated by IgG2a/IgG1 ratios, which were equivalent or higher for TRYP/Vec-vaccinated mice than for TRYP/TRYP-vaccinated mice, the IFN-γ/IL-10 ratios in LN cells were significantly higher (P < 0.01) for TRYP/TRYP-vaccinated mice (52.0 ± 5.55) than for TRYP/Vec-vaccinated mice (35.9 ± 2.39), indicating that Treg is a possible source of IL-10. Nevertheless, the ratio of IFN-γ to IL-10 for the draining LN recall response was clearly sufficient in this effector phase of the postvaccination response to mediate protection whether mice had been vaccinated with DNA alone or heterologous DNA/MVA priming-boosting.
TRYP/TRYP is more protective than TRYP/Vec when mice are challenged with L. major 16 weeks after booster vaccination.
Given that TRYP/Vec was as protective as TRYP/TRYP at 2 weeks after booster vaccination and immune responses in draining LN suggested that little enhanced efficacy was provided by boosting with TRYP MVA, the length of protection was determined by examining the responses to challenge infection at 16 weeks after booster vaccination. MVA is a nonreplicating virus; therefore, this time frame represents immunological memory as opposed to the effector phase of the immune response at 2 weeks postchallenge. As previously shown at 2 weeks after booster vaccination, TRYP/TRYP significantly protected (P < 0.001) mice from leishmaniasis compared to Vec/Vec-vaccinated mice at 1, 2, and 3 weeks postchallenge (Fig. 5A), after which euthanasia was necessary for Vec/Vec-vaccinated mice. In contrast, TRYP/Vec provided protection during weeks 1 and 2 after L. major challenge, but the protective effect waned by week 3 (Fig. 5B). A comparison of the TRYP/TRYP and TRYP/Vec data (Fig. 5C) demonstrated that significant enhancement of the efficacy resulted from boosting with TRYP MVA (P < 0.01). Hence, the potent IFN-γ response measured in splenocytes during the effector phase 2 weeks after booster vaccination may have affected the clinical phenotype when mice were challenged 16 weeks after boosting with TRYP MVA, possibly reflecting early induction of a central memory response.
FIG. 5.
Clinical outcome following L. major challenge of BALB/c mice vaccinated with TRYP/TRYP, TRYP/Vec, or Vec/Vec at 16 weeks after booster vaccination. Mice were vaccinated with two 100-μg doses of TRYP or vector DNA given 3 weeks apart and boosted with 1 × 106 PFU of TRYP or vector MVA 2 weeks later. The mice were challenged at 16 weeks after booster administration with 2 × 106 L. major promastigotes in the hind footpad, and the footpad depth was monitored weekly with vernier calipers. The results are means ± standard errors for groups of mice in one experiment and are representative of the results of replicate experiments in which similar results were obtained. The asterisks indicate significant differences as determined by Student's t test (one asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001).
TRYP/TRYP elicits more potent long-term immunological memory with Th1 bias than TRYP/Vec elicits.
Serum immunoglobulin levels were determined 16 weeks after booster vaccination either before (Fig. 6A and C) or after (Fig. 6B and D) L. major challenge. As previously demonstrated at 2 weeks after booster vaccination (Fig. 2), the IgG1 and IgG2a responses were similar in TRYP/TRYP-vaccinated mice and TRYP/Vec-vaccinated mice, and the IgG2a/IgG1 ratios suggested that there was a greater bias toward Th1 in TRYP/Vec-vaccinated animals (1.3) than in TRYP/TRYP-vaccinated animals (0.51). In contrast, the IgG2a/IgG1 ratios after challenge indicated that there was a greater bias toward Th1 in TRYP/TRYP-vaccinated mice (1.97) than in TRYP/Vec-vaccinated mice (1.06) and Vec/Vec-vaccinated mice (0.05). These findings reflect the significantly higher IgG2a levels in both TRYP/TRYP-vaccinated mice and TRYP/Vec-vaccinated mice than in Vec/Vec-vaccinated mice after challenge, which, measured against similar IgG1 levels, demonstrated that a clear Th1 bias was elicited by immunization with TRYP per se.
FIG. 6.
TRYP-specific IgG1 and IgG2a responses in mice vaccinated with TRYP/TRYP, TRYP/Vec, or Vec/Vec before and after L. major challenge. Mice were vaccinated with two 100-μg doses of TRYP or vector DNA given 3 weeks apart and boosted with 1 × 106 PFU of TRYP or vector MVA 2 weeks later. At 16 weeks after booster administration, either immune cells were recalled by in vivo injection of 5 μg FTP in the hind footpad and serum was removed after 48 h (A and C) or mice were challenged in the hind footpad with 2 × 106 L. major promastigotes and serum was removed 2 weeks later (B and D). TRYP-specific IgG1 (A and B) and IgG2a (C and D) were analyzed by ELISA using pooled sera. The antibody levels are expressed as averages for duplicate absorbance (OD) determinations for 12 serial twofold dilutions. The dotted line indicates the value used to determine end point titers. The results are representative of the results of replicate experiments in which similar results were obtained.
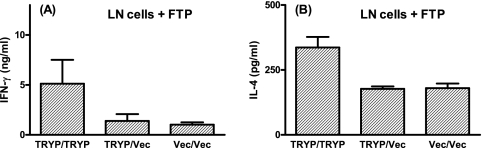
The in vitro restimulation of draining LN cells with FTP at 16 weeks after booster vaccination demonstrated that there was retention of antigen-specific cytokine responses in TRYP/TRYP-vaccinated mice but not inTRYP/Vec-vaccinated mice (Fig. 7). The FTP IFN-γ responses were similar to those observed at 2 weeks postchallenge in the TRYP/TRYP group (compare Fig. 7A with Fig. 4B), demonstrating that MVA boosting elicited a sustained T-cell memory response that was consistent with improved efficacy of the heterologous prime-boost vaccine strategy upon clinical challenge (Fig. 5). The cytokine responses in draining LN cells measured after L. major challenge also demonstrated that there was a clear bias toward Th1 for TRYP/TRYP compared to TRYP/Vec (Table 1). Significantly elevated ratios of IFN-γ to IL-10 and of IFN-γ to IL-4 were observed for TRYP/TRYP-vaccinated mice compared to TRYP/Vec-vaccinated mice (P < 0.001) (Table 1). Higher levels of the Th1 cytokine IFN-γ than of the immunoregulatory Th2/Treg cytokines, such as IL-4 and IL-10, coupled with the demonstration of a more Th1-biased immunoglobulin response, demonstrated that heterologous DNA/MVA prime-boost vaccines are likely to be more efficacious than immunization with DNA alone in the clinical setting.
FIG. 7.
FTP-mediated IFN-γ and IL-4 responses in LN cells derived from BALB/c mice immunized with TRYP/TRYP, TRYP/Vec, or Vec/Vec. At 16 weeks after booster vaccination, in vivo recall was performed by footpad injection of 5 μg FTP 48 h prior to removal of draining LN cells. Triplicate wells containing pooled LN cells were restimulated in vitro with 10 μg/ml FTP for 72 h, and supernatants were removed for detection of IFN-γ or IL-4 by ELISA. The results (means ± standard errors) are representative of replicate experiments in which similar results were obtained.
TABLE 1.
Postchallenge measurement of Th1-Th2/Treg in mice challenged 16 weeks after booster vaccination
| Vaccine | Ratiosa
|
||
|---|---|---|---|
| IFN-γ/IL-10 | IFN-γ/IL-4 | IgG2a/IgG1 | |
| TRYP/TRYP | 37.8 ± 2.6 | 402 ± 16.9 | 1.97 |
| TRYP/Vec | 25.8 ± 1.6 | 297 ± 14.0 | 1.06 |
The ratios of IFN-γ to IL-10 and of IFN-γ to IL-4 for TRYP/TRYP- and TRYP/Vec-vaccinated mice were compared using Student's t test, and the P values were both <0.001.
DISCUSSION
We (29) and other workers (3) have shown previously that susceptible BALB/c mice vaccinated with DNA encoding the TRYP antigen are protected against infection with L. major when mice are challenged during the effector phase of the postvaccination immune response. We also demonstrated previously (29) that heterologous priming-boosting with TRYP/TRYP DNA/MVA protected mice challenged in the effector phase. A crucial question in deciding which of these vaccination strategies might be more efficacious as a clinical vaccine was whether the strategies differ in the ability to elicit a long-term memory response. Here we demonstrated that heterologous priming-boosting with TRYP/TRYP DNA/MVA elicited a more sustained Th1-biased immune response than immunization with DNA alone elicited, and there was an improved clinical outcome when mice were challenged in the later memory phase of the postvaccination immune response. Together with concerns about the recognition of DNA in humans, in which Toll-like receptor 9 (TLR9) expression is restricted to B cells and plasmacytoid dendritic cells (30), these results suggest that a heterologous prime-boost strategy might provide a way forward in the development of a vaccine for human clinical trials.
Examination of immune responses in draining LN 2 weeks after administration of MVA demonstrated that the levels of IFN-γ in TRYP/Vec- and TRYP/TRYP-vaccinated mice were equivalent, suggesting that there were comparable effector memory CD4+ T cell responses when the two immunization regimens were used. However, the splenocyte TRYP- and FTP-specific IFN-γ responses were significantly higher in the TRYP/TRYP-vaccinated group. Based on evidence that central memory CD4+ T cells mediate long-term immunity to L. major (36), it is possible that IFN-γ-producing CD4+ T cells retained in the spleen represent central memory cells rather than effector memory cells, which are subsequently liberated upon in vivo recall at 16 weeks. This correlates with the enhanced levels of protection in TRYP MVA-boosted mice during challenge at 16 weeks after booster vaccination. While central memory cells divide in response to antigen stimulation, they are associated more with IL-2 production than with IFN-γ production (35, 36). It is possible that the in vivo FTP recall, followed by in vitro restimulation with antigen, was sufficient for differentiation of central memory T cells in the spleen into IFN-γ-producing effector memory cells. Further characterization using cell surface markers specific for central memory T cells (including CD44, CD62L, and pre-Th1-associated chemokines such as CXCR3 [28]), as well as in vivo cell transfers, would be informative for determining the phenotype of cells elicited early in the spleens of MVA-boosted mice.
Another question that arises is why heterologous priming-boosting with DNA followed by MVA is more effective for generating long-term memory than priming-boosting with DNA alone is. We showed that DNA/MVA was more Th1 polarizing, especially during the memory response, although there was evidence that TRYP/TRYP-vaccinated mice produced more IFN-γ following exposure to live L. major parasites during the effector phase. One explanation is that more sustained memory responses may have resulted from the differential activation of DC subsets by DNA and MVA. For example, CD8α+ DC that produce IL-12 and IFN-γ have been associated with Th1 polarization, whereas CD8α− DC in the presence of IL-10 are associated with Th2 (17, 18). Furthermore, CD8α+ DC are involved in priming cytotoxic T lymphocyte immunity to viruses, including vaccinia virus (1). An alternative hypothesis is that the specific interaction of pathogen-derived molecules with distinct TLRs expressed on DC subsets defines Th1/Th2 polarization (2, 21). For example, the interaction of CpG with TLR9 but not the interaction of lipopolysaccharide with TLR4 on plasmacytoid DC induces the development of Th1 (2). Recent evidence has shown that TLR3 and TLR4 potently act synergistically with TLR7, TLR8, and TLR9 in the induction of Th1-polarizing genes (21). Other factors influencing T-cell polarization include antigen dose and signal strength. However, there is little information concerning the critical factors necessary for induction of potent Th1-polarized CD4+ central memory cells, which will be critical for the development of vaccines against intracellular pathogens, such as leishmania.
While we show here that heterologous priming-boosting with DNA followed by MVA expressing TRYP elicited sustained protective immunity to leishmaniasis, we previously demonstrated that a similar immunization regimen utilizing a Leishmania homologue of the receptor for activated C kinase did not provide protection (34). Similarly, a comparison of the cytokine responses during the memory phase revealed diminished responses for LACK/Vec compared to the responses for LACK/LACK, although LACK was not protective as DNA alone or DNA/MVA at either 2 or 16 weeks after booster vaccination (data not shown). These data demonstrate the importance of both the immunization regimen employed and the microbial antigen used in the generation of appropriate protective immunity to L. major. Other laboratories have shown that LACK is protective both as DNA (8-10) and upon heterologous priming-boosting with DNA followed by MVA (7), although LACK did not provide protection against Leishmania mexicana (6) and visceral leishmaniasis (20). The findings obtained for the Friedlin and WHOM/IR/-173 substrains of L. major by previous workers (7-10), compared to the findings for the potent substrain LV39 used here, might also explain why LACK induced protection. Noben-Trauth et al. (22, 23) demonstrated that there were clear differences in immune requirements in susceptible BALB/c mice depending on the L. major challenge substrain; LV39 was less susceptible to IFN-γ-mediated killing than IR173, and both of these substrains were less susceptible than the Friedlin substrain (22).
Using L. major substrain IR173, Gonzalo et al. (7) observed enhanced immune responses and clinical efficacy after boosting LACK DNA-vaccinated mice with LACK-expressing vaccinia virus at 3 weeks after booster immunization. The immune responses were examined in spleens but not in draining LN, which showed that there were enhanced IFN-γ levels after LACK MVA boosting, as we observed here for TRYP. Rather than replication-incompetent MVA, Gonzalo utilized the replication-competent Western Reserve strain of vaccinia virus, which influenced the longevity of viral expression in target organs and subsequent immunogenicity, especially with respect to cross-reacting viral antigens (24, 25). A recent comparison of heterologous boosting with the Western Reserve strain and boosting with MVA expressing LACK in a visceral leishmaniasis model revealed that there were more potent Th1-Th2/Treg (IFN-γ-IL-10) responses for the Western Reserve strain before challenge, although the levels of protection were equivalent for the two viral strains (5).
In our study we did not demonstrate that CD8+ T cells were important in our vaccine model, whereas previous evidence obtained using DNA vaccination with LACK demonstrated that CD8+ T cells have a role both as effector cells and in maintaining the frequency of CD4+ IFN-γ-producing T cells (10). In addition, CD8+ T cells induced long-term memory in response to heat-killed Leishmania antigen or recombinant protein plus CpG oligonucleotides (27), which was dependent on the interaction of CpG with CD11c+ DC (33). Importantly, immunization with the LACK protein plus IL-12 provided short-term protection that correlated with the inability to elicit antigen-specific CD8+ T cells. Hence, it was suggested that activation of CD8+ T cells was essential for the maintenance of CD4+ T-cell memory. It is highly probable that in vitro stimulation of immune cells with recombinant TRYP protein in our model failed to target IFN-γ production by CD8+ T cells, and this would be better demonstrated using DC infected with live parasites or an appropriate peptide. Indeed, stimulation of LN cells from TRYP/TRYP-vaccinated mice with L. major-infected DC revealed enhanced IFN-γ production compared to that in TRYP/Vec-vaccinated mice, which could have resulted from more appropriate targeting of CD8+ T cells. It is also possible that TRYP MVA-mediated CD8+ T cells are critical for generation of CD4+ central memory cells and for maintenance of long-term immunity to leishmaniasis. Further work is required to test this hypothesis.
In conclusion, we demonstrated that heterologous priming-boosting with TRYP DNA/TRYP MVA promoted long-term immunity to L. major that was more robust than the protection provided by TRYP DNA alone. This enhanced protection correlated with sustained Th1 immunity to L. major infection in the TRYP/TRYP-vaccinated group.
Acknowledgments
We thank M. Saraiva and L. Burrows for help with the generation of vaccinia virus recombinants.
This work was supported by a studentship to U.G.L. from the Elmore Trust and the James Baird Fund, by a fellowship to M.T.M.R. from the Addenbrooke's Hospital Trust, and by a program grant to J.M.B. from the British Medical Research Council. A.A. was funded by The Wellcome Trust.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Belz, G. T., C. M. Smith, D. Eichner, K. Shortman, G. Karupiah, F. R. Carbone, and W. R. Heath. 2004. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J. Immunol. 172:1996-2000. [DOI] [PubMed] [Google Scholar]
- 2.Boonstra, A., C. Asselin-Paturel, M. Gilliet, C. Crain, G. Trinchieri, Y. J. Liu, and A. O'Garra. 2003. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential Toll-like receptor ligation. J. Exp. Med. 197:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos-Neto, A., J. R. Webb, K. Greeson, R. N. Coler, Y. A. Skeiky, and S. G. Reed. 2002. Vaccination with plasmid DNA encoding TSA/LmSTI1 leishmanial fusion proteins confers protection against Leishmania major infection in susceptible BALB/c mice. Infect. Immun. 70:2828-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davison, A. J., and B. Moss. 1990. New vaccinia virus recombination plasmids incorporating a synthetic late promoter for high level expression of foreign proteins. Nucleic Acids Res. 18:4285-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondji, B., E. Perez-Jimenez, K. Goldsmith-Pestana, M. Esteban, and D. McMahon-Pratt. 2005. Heterologous prime-boost vaccination with the LACK antigen protects against murine visceral leishmaniasis. Infect. Immun. 73:5286-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumonteil, E., R. S. Maria Jesus, E. O. Javier, and G. M. Maria del Rosario. 2003. DNA vaccines induce partial protection against Leishmania mexicana. Vaccine 21:2161-2168. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalo, R. M., G. del Real, J. R. Rodriguez, D. Rodriguez, R. Heljasvaara, P. Lucas, V. Larraga, and M. Esteban. 2002. A heterologous prime-boost regime using DNA and recombinant vaccinia virus expressing the Leishmania infantum P36/LACK antigen protects BALB/c mice from cutaneous leishmaniasis. Vaccine 20:1226-1231. [DOI] [PubMed] [Google Scholar]
- 8.Gurunathan, S., C. Prussin, D. L. Sacks, and R. A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409-1415. [DOI] [PubMed] [Google Scholar]
- 9.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 186:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurunathan, S., L. Stobie, C. Prussin, D. L. Sacks, N. Glaichenhaus, A. Iwasaki, D. J. Fowell, R. M. Locksley, J. T. Chang, C. Y. Wu, and R. A. Seder. 2000. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells. J. Immunol. 165:915-924. [DOI] [PubMed] [Google Scholar]
- 11.Heinzel, F. P., M. D. Sadick, B. J. Holaday, R. L. Coffman, and R. M. Locksley. 1989. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 169:59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julia, V., E. M. Hessel, L. Malherbe, N. Glaichenhaus, A. O'Garra, and R. L. Coffman. 2002. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity 16:271-283. [DOI] [PubMed] [Google Scholar]
- 13.Launois, P., I. Maillard, S. Pingel, K. G. Swihart, I. Xenarios, H. Acha-Orbea, H. Diggelmann, R. M. Locksley, H. R. MacDonald, and J. A. Louis. 1997. IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 6:541-549. [DOI] [PubMed] [Google Scholar]
- 14.Levick, M. P., J. M. Blackwell, V. Connor, R. M. Coulson, A. Miles, H. E. Smith, K. L. Wan, and J. W. Ajioka. 1996. An expressed sequence tag analysis of a full-length, spliced-leader cDNA library from Leishmania major promastigotes. Mol. Biochem. Parasitol. 76:345-348. [DOI] [PubMed] [Google Scholar]
- 15.Levick, M. P., E. Tetaud, A. H. Fairlamb, and J. M. Blackwell. 1998. Identification and characterisation of a functional peroxidoxin from Leishmania major. Mol. Biochem. Parasitol. 96:125-137. [DOI] [PubMed] [Google Scholar]
- 16.Locksley, R. M., and P. Scott. 1991. Helper T-cell subsets in mouse leishmaniasis: induction, expansion and effector function. Immunol. Today 12:A58-A61. [DOI] [PubMed] [Google Scholar]
- 17.Maldonado-Lopez, R., T. De Smedt, P. Michel, J. Godfroid, B. Pajak, C. Heirman, K. Thielemans, O. Leo, J. Urbain, and M. Moser. 1999. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maldonado-Lopez, R., C. Maliszewski, J. Urbain, and M. Moser. 2001. Cytokines regulate the capacity of CD8alpha(+) and CD8alpha(−) dendritic cells to prime Th1/Th2 cells in vivo. J. Immunol. 167:4345-4350. [DOI] [PubMed] [Google Scholar]
- 19.Mauel, J. 2002. Vaccination against Leishmania infections. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2:201-226. [DOI] [PubMed] [Google Scholar]
- 20.Melby, P. C., J. Yang, W. Zhao, L. E. Perez, and J. Cheng. 2001. Leishmania donovani p36(LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis. Infect. Immun. 69:4719-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Napolitani, G., A. Rinaldi, F. Bertoni, F. Sallusto, and A. Lanzavecchia. 2005. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 6:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noben-Trauth, N., R. Lira, H. Nagase, W. E. Paul, and D. L. Sacks. 2003. The relative contribution of IL-4 receptor signaling and IL-10 to susceptibility to Leishmania major. J. Immunol. 170:5152-5158. [DOI] [PubMed] [Google Scholar]
- 23.Noben-Trauth, N., W. E. Paul, and D. L. Sacks. 1999. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J. Immunol. 162:6132-6140. [PubMed] [Google Scholar]
- 24.Ramirez, J. C., M. M. Gherardi, and M. Esteban. 2000. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J. Virol. 74:923-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez, J. C., M. M. Gherardi, D. Rodriguez, and M. Esteban. 2000. Attenuated modified vaccinia virus Ankara can be used as an immunizing agent under conditions of preexisting immunity to the vector. J. Virol. 74:7651-7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiner, S. L., Z. E. Wang, F. Hatam, P. Scott, and R. M. Locksley. 1993. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science 259:1457-1460. [DOI] [PubMed] [Google Scholar]
- 27.Rhee, E. G., S. Mendez, J. A. Shah, C. Y. Wu, J. R. Kirman, T. N. Turon, D. F. Davey, H. Davis, D. M. Klinman, R. N. Coler, D. L. Sacks, and R. A. Seder. 2002. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against leishmania major infection. J. Exp. Med. 195:1565-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivino, L., M. Messi, D. Jarrossay, A. Lanzavecchia, F. Sallusto, and J. Geginat. 2004. Chemokine receptor expression identifies pre-T helper (Th)1, pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J. Exp. Med. 200:725-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts, M. T. M., C. Stober, A. N. McKenzie, and J. M. Blackwell. 2005. IL-4 and IL-10 collude in vaccine failure for novel exacerbatory antigens in murine Leishmania major infection. Infect. Immun. 73:7620-7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothenfusser, S., E. Tuma, S. Endres, and G. Hartmann. 2002. Plasmacytoid dendritic cells: the key to CpG. Hum. Immunol. 63:1111-1119. [DOI] [PubMed] [Google Scholar]
- 31.Scott, P., P. Natovitz, R. L. Coffman, E. Pearce, and A. Sher. 1988. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. Exp. Med. 168:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott, P., E. Pearce, A. W. Cheever, R. L. Coffman, and A. Sher. 1989. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol. Rev. 112:161-182. [DOI] [PubMed] [Google Scholar]
- 33.Shah, J. A., P. A. Darrah, D. R. Ambrozak, T. N. Turon, S. Mendez, J. Kirman, C. Y. Wu, N. Glaichenhaus, and R. A. Seder. 2003. Dendritic cells are responsible for the capacity of CpG oligodeoxynucleotides to act as an adjuvant for protective vaccine immunity against Leishmania major in mice. J. Exp. Med. 198:281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stober, C. B., U. G. Lange, M. T. Roberts, A. Alcami, and J. M. Blackwell. 2005. IL-10 from regulatory T cells determines vaccine efficacy in murine Leishmania major infection. J. Immunol. 175:2517-2524. [DOI] [PubMed] [Google Scholar]
- 35.Wu, C. Y., J. R. Kirman, M. J. Rotte, D. F. Davey, S. P. Perfetto, E. G. Rhee, B. L. Freidag, B. J. Hill, D. C. Douek, and R. A. Seder. 2002. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat. Immunol. 3:852-858. [DOI] [PubMed] [Google Scholar]
- 36.Zaph, C., J. Uzonna, S. M. Beverley, and P. Scott. 2004. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat. Med. 10:1104-1110. [DOI] [PubMed] [Google Scholar]