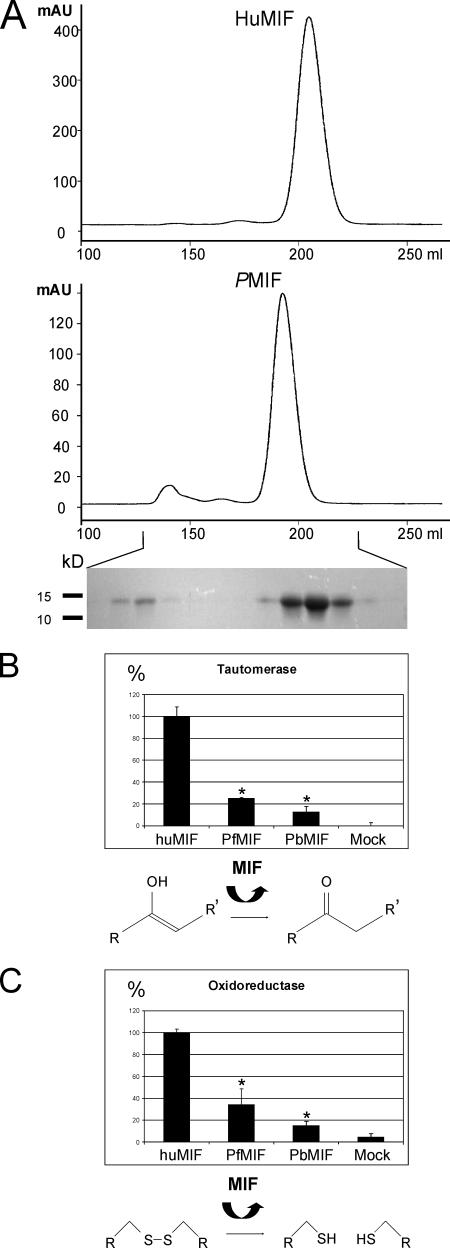
FIG. 5.
Recombinant PMIF elutes as a dimer in size exclusion chromatography and is active in tautomerase and oxidoreductase assays. (A) Chromatograms of C-terminally His6-tagged huMIF, which resulted in calculated molecular masses of 30 kDa for PbMIF and 22 kDa for huMIF. The reference compounds included bovine albumin (66 kDa; 153.93 ml), chicken ovalbumin (45 kDa; 170.93 ml), bovine carbonic anhydrase (30 kDa; 192.47 ml), and bovine α-lactalbumin (14.4 kDa; 217.39 ml). The inset shows a Coomassie blue-stained protein gel containing the peak fractions for PbMIF, which identified the void volume peak as PbMIF aggregates. The same results were obtained with PfMIF (data not shown). mAU, milli-absorbance units. (B and C) Recombinant MIF tautomerase activity with p-hydroxyphenylpyruvate (R = COOH and R′ = C6H4-OH) (B) and oxidoreductase activity with 2-hydroxyethyldisulfide (R = CH2-OH) (C). Sample equations are shown for both conversions. The activities of PfMIF and PbMIF are expressed as percentages of the huMIF activity. In both sets of experiments, the PfMIF and PbMIF activities were greater than the activities of the mock purification control. Asterisks indicate that the P value is <0.05 (n ≥ 3).