Abstract
The persistence and transmission of Aeromonas in a duckweed aquaculture-based hospital sewage water treatment plant in Bangladesh was studied. A total of 670 samples from different sites of the hospital sewage water treatment plant, from feces of hospitalized children suffering from diarrhea, from environmental control ponds, and from feces of healthy humans were collected over a period of three years. In total, 1,315 presumptive Aeromonas isolates were biochemically typed by the PhenePlate rapid screening system (PhP-AE). A selection of 90 representative isolates was further analyzed with PhenePlate (PhP) extended typing (PhP-48), fatty acid methyl ester analysis, and amplified fragment length polymorphism (AFLP) fingerprinting. In addition, the prevalence of the putative virulence factors hemolysin and cytotoxin and the presence of the cytolytic enterotoxin gene (AHCYTOEN) were analyzed. Aeromonas was found at all sites of the treatment plant, in 40% of the samples from environmental control ponds, in 8.5% of the samples from hospitalized children suffering from diarrhea, and in 3.5% of samples from healthy humans. A significantly high number of Aeromonas bacteria was found in duckweed, which indicates that duckweed may serve as a reservoir for these bacteria. PhP-AE typing allowed identification of more than 192 distinct PhP types, of which 18 major PhP types (MTs) were found in multiple sites and during several occasions. AFLP fingerprinting revealed the prevalence of genotypically indistinguishable Aeromonas isolates among certain PhP MTs recovered from different sampling occasions and/or at multiple sites. Hemolytic and cytotoxic activities were observed in 43% of the tested strains, whereas 29% possessed the cytolytic enterotoxin gene AHCYTOEN. Collectively, two specific MTs associated with diarrhea were shown to exhibit high cytotoxicity. Furthermore, all tested isolates of these major types were positive for the cytolytic enterotoxin gene. In conclusion, our data indicate that certain phenotypically and genotypically stable clonal lineages of Aeromonas have persisted in the treatment system for a prolonged period and might spread from the hospitalized children suffering from diarrhea to fish produced for human consumption through the sewage water treatment system.
In many developing countries, freshwater reserves are decreasing due to growing populations, increased human consumption, urbanization, and the lack of cost-effective sewage water treatment systems (6, 27). To address these problems, it has been proposed that treated sewage water with a low level of chemical and microbiological contamination be used directly or indirectly for domestic, industrial, aquaculture, and agricultural purposes (6, 25). A relatively new method that indirectly uses sewage water in aquaculture is the duckweed aquaculture-based sewage water treatment system. Such sewage water treatment systems are becoming common practice in different Southeast Asian countries, including Thailand, Malaysia, Taiwan, and Bangladesh, as well as in China and the Unites States (1, 31, 51). The use of the free-floating duckweed in sewage water treatment systems has been advocated because it is fast growing and easy to harvest, has a low fiber and high protein content, and shows a high efficiency in removal of nitrogen and phosphorous from sewage water (31). In this way, sewage water can be transferred into high-quality fish feed instead of being disposed of in the environment. The growing interest also seems to be due to the potential cost recovery through the use of duckweed as animal feed (13, 35). However, sewage water recycling may constitute a health hazard, since sewage waters are known to be potential reservoirs of pathogenic and/or drug-resistant bacteria and other pathogenic organisms (5, 28) that may persist in the sewage treatment system, be recycled through the reuse of treated sewage water, and possibly spread to humans through the food chain (23, 40).
Bacteria of the coliform group, in particular thermotolerant Escherichia coli, have long been used as indicators of fecal contamination. E. coli, as well as other species of bacteria present in sewage water, may cause gastroenteritis and other infections in humans and animals (10). The primary habitat of E. coli is the lower intestine of warm-blooded animals, and they have a low survival rate outside animal hosts in comparison to other environmental bacteria, such as Aeromonas (48). The present study focused on the freshwater organism Aeromonas, which is widely distributed in the environment and has been isolated from soil, marine water, fresh water, sewage water, drinking water, food, salad, ready-to-eat food, smoked salmon, and vegetables (3, 14, 36). The genus Aeromonas is known to comprise opportunistic pathogens associated with gastroenteritis, bacteremia, and wound infections in humans but also with soft-tissue infections in fish (24). It has also been shown that some subgroups or clonal groups of these species are associated with human and fish infection and probably represent pathogenic Aeromonas lineages (32, 33, 42). Aeromonads have been found to persist for a prolonged period in a drinking-water well (34) or in a water microcosm (37), which favors the possible transmission of these organisms to humans. Because of this remarkable survival potential, it is possible that insufficient treatment and/or recycling of sewage water may facilitate persistence and dissemination of potentially pathogenic subgroups or clonal groups of Aeromonas throughout aquatic and human environments.
The aim of the present study was to investigate the persistence and the possibility of transmission of Aeromonas strains from hospital sewage water, via a duckweed aquaculture-based sewage water treatment system, to fish for human consumption. In addition, we also studied the prevalence of putative virulence factors of a selection of Aeromonas strains that were recovered from multiple sampling sites along the recycling system.
MATERIALS AND METHODS
Sampling sites and sample collection.
The study was conducted with a model system of duckweed aquaculture-based hospital sewage water treatment plant (Kumudini hospital and its residential area complex, located in Tangail, Bangladesh) during 2000 to 2002. In the treatment plant, hospital raw sewage water is first collected in a settlement pond (referred to as sewage water in this study) and is then transferred to a lagoon, where the duckweed (Lemnaceae) is grown (referred to as a duckweed lagoon). The duckweed is harvested and used as feed for the fish in a separate pond (referred to as a fish pond). During three consecutive years, a total of 670 samples were collected during the months of December to February. Samples were collected at various stages of the sewage treatment process (untreated sewage water, duckweed lagoon, and fish pond) and from the duckweed grown in the lagoon, from harvested duckweed used as fish feed, and from fish (only tilapia [Oreochromis nilotica]) skin lesions, gills, intestines, and kidneys. Samples (water, sediment, and duckweed) were collected from the above-mentioned sites in sterile bottles using aseptic techniques. For water, sediment, and duckweed, four subsamples were collected at different places and pooled together. Control samples were collected from water and sediment in ponds used for household use and/or fish culture in a nearby area (referred to as control ponds). Fecal samples were obtained from hospitalized children up to 5 years of age with acute diarrhea at the Diarrheal Service Centre in Kumudini Hospital Complex (referred to as child diarrhea). Fecal samples from healthy adult human subjects living in the adjacent area were also collected (referred to as healthy humans). All samples were transported to the laboratory at 4 to 8°C and analyzed within 18 h after collection.
Sample processing.
Aeromonas selective agar base (Havelaar) without antibiotics was used for isolation of mesophilic Aeromonas (Biolife, Milano, Italy). Water samples (10 or 100 μl) were usually streaked directly on the agar plates, except for samples with presumed low counts of bacteria, of which 1 to 10 ml was first filtered through 0.45-μm membranes (Millipore, Billerica), which were then incubated on agar plates. For solid samples, 10 g of sediment, duckweed, or fish sample was mixed with 10 ml phosphate-buffered saline and vigorously shaken at room temperature for 30 min. Ten or one hundred microliters of this raw sample was then inoculated on the agar plates. Samples with presumed high counts of bacteria were subjected to serial dilutions before being streaked on appropriate agar plates. Human fecal samples (0.5 g) were enriched in 5 ml of bile peptone broth for 12 h at 37°C and then streaked onto Aeromonas selective agar plates.
Bacteriological analysis.
Aeromonas isolates recovered from selective agar were subcultured on brain heart infusion agar plates at 37°C overnight. The isolates were tested for positive oxidase reactions and resistance to 150 μg/ml of the vibriostatic agent O/129 and were thereafter enumerated. Eight positive colonies from each sample were selected for typing with the PhenePlate (PhP) extended typing (PhP-AE) system, except for three diarrheal stool samples, where 16 colonies were selected.
Biochemical fingerprinting with the PhP system.
The isolates were typed by a biochemical phenotyping method, the PhP-AE system (PhPlate Microplate Techniques AB, Stockholm, Sweden), according to the manufacturer's instructions (39). The PhP fingerprints of the isolates were compared pairwise, and the similarity between each pair was expressed as a correlation coefficient. Isolates were assigned to the same PhP type when they showed a correlation coefficient higher then 0.975. The diversities among populations were calculated as Simpson's diversity index (Di), and clustering of the isolated was performed using the unweighted pair group method with arithmetic means method (45). PhP types containing at least two isolates from the same sample were referred to as common types, while those containing only one isolate were referred to as single types. One isolate per sample belonging to each common type was stored in brain heart infusion broth with 30% glycerol at −70°C. After data analysis, 90 selected isolates representing common types were reanalyzed with the more discriminatory typing system PhP-48 (39), in which 48 instead of 11 fermentation reactions were used.
Identification of Aeromonas by FAME and AFLP analysis.
The same 90 selected Aeromonas strains representing each common types were identified to the genomic species level by using gas-liquid chromatographic analysis of cellular fatty acid methyl esters (FAMEs) as described previously (17). A selection of 46 isolates was further subjected to whole-genome fingerprinting using amplified fragment length polymorphism (AFLP) analysis according to the protocol described by Huys and Swings (15). The AFLP profiles of unknown isolates were compared with the laboratory-based identification library AEROLIB, comprising AFLP profiles generated from a collection of well-characterized type and reference strains encompassing all currently recognized Aeromonas taxa (18).
Cytotoxic and hemolysin activity.
Cytotoxic activity of the 90 isolates, representing 90 common types, was tested on HEp-2 cell lines (human epithelial cells) as described previously (12). Briefly, confluent monolayers of the cells were grown in 24-well tissue culture plates (Coster, Corning, NY) in minimal essential medium supplemented with 10% fetal bovine serum, 1% (wt/vol) glutamine, and 1% (wt/vol) penicillin-streptomycin. One hundred microliters of sterile culture supernatant (containing 150 mg liter−1 protein as determined by Bio-Rad protein assay) were serially twofold diluted in supplemented minimal essential medium and incubated for 6 h at 37°C with the HEp-2 cells. Cytotoxic activity was measured as rounding, detachment, and loss of viability of the cells, as seen with the light microscope after 6 h. The titer was determined as the highest dilution of the supernatant affecting at least 50% of the cells. Isolates showing a cytotoxic effect at a dilution of 1/8 or more were regarded as cytotoxin positive.
Hemolytic activities of the same isolates were measured using 1% (vol/vol) rabbit erythrocytes as described earlier (26). Isolates were considered positive for hemolysin production when the culture supernatant at a final concentration of 1/8 lysed at least 50% of the erythrocytes upon visual inspection. Strains BD2-9 and BD12, originating from a previous study, were included as positive and negative controls, respectively, for both the cytotoxin and hemolysin assays (42). To compare the mean cytotoxic titer within the PhP types, the data were subjected to statistical evaluation by using the t test.
PCR detection of cytolytic enterotoxin and/or extracellular hemolysin gene.
The same 90 isolates, representing 90 common types, were screened for the presence of the cytolytic enterotoxin gene and/or the extracellular hemolysin gene using a PCR-based assay described previously (42). PCR amplicons were separated electrophoretically in a 1.5% agarose gel (Sigma type 1) and visualized after ethidium bromide staining. Positive and negative controls from a previous study were included during each PCR experiment (42).
RESULTS
Occurrence of Aeromonas in different samples.
The isolation frequencies and enumeration data for presumptive Aeromonas based on the analysis of 670 samples are shown in Tables 1 and 2. All samples (n = 86) from the sewage water treatment plant but only 27 out of the 68 (40%) samples from the control ponds were positive for Aeromonas. Likewise, Aeromonas was also isolated from 19 (8.5%) diarrheal children and from 9 (3.4%) healthy humans. The highest mean number of Aeromonas bacteria was found in the untreated sewage sediment, followed by the duckweed (both in the lagoon and in the duckweed used as fish feed), the sediments (in the lagoon and in the fish pond), and the water in the treatment plants. The lowest mean numbers of Aeromonas were generally found in the control ponds. A significantly higher number of Aeromonas bacteria were present in sewage water than in the control ponds (P < 0.001).
TABLE 1.
Occurrence and diversity of Aeromonas bacteria found in different sites of the sewage treatment plant or in diarrheal children, healthy humans, or control ponds
| Sampling site (na) | No. (%) of samples positive for Aeromonas | Amt of Aeromonas [mean (median) CFU/ml or CFU/g] | No. of isolates analyzed by PhP | Diversity index (mean ± SE)b | No. of common PhP types |
|---|---|---|---|---|---|
| Sewage water | 31 | ||||
| Water (18) | 18 (100) | 3.7 × 104 (8,500) | 144 | 0.96 ± 0.01 | |
| Sediment (8) | 8 (100) | 2.7 × 106 (5,33,500) | 64 | 0.92 ± 0.03 | |
| Duckweed lagoon | 38 | ||||
| Water (10) | 10 (100) | 1.2 × 104 (3,850) | 80 | 0.93 ± 0.02 | |
| Sediment (8) | 8 (100) | 1.2 × 105 (8,300) | 64 | 0.92 ± 0.03 | |
| Duckweed (12) | 12 (100) | 1.9 × 106 (17,00,000) | 96 | 0.89 ± 0.03 | |
| Fish culture pond | 34 | ||||
| Water (12) | 12 (100) | 2.4 × 104 (8,450) | 96 | 0.91 ± 0.02 | |
| Sediment (11) | 11 (100) | 1.5 × 105 (56,000) | 88 | 0.89 ± 0.03 | |
| Duckweed (7) | 7 (100) | 1.4 × 106 (13,00,000) | 56 | 0.89 ± 0.04 | |
| Fish: gill, lesion, intestine (29) | 29 (100) | NAc | 231 | 0.93 ± 0.01 | 34 |
| Control pond: water, sediment (68) | 27 (40) | 5.3 × 101 (NA) | 166 | 0.97 ± 0.01 | 27 |
| Human feces | |||||
| Diarrheal child (222) | 19 (9) | NA | 167 | 0.83 ± 0.02 | 16 |
| Healthy human (265) | 9 (3) | NA | 63 | 0.87 ± 0.04 | 12 |
n, no. of samples analyzed.
Simpson's index.
NA, not analyzed.
TABLE 2.
Numbers of presumptive Aeromonas bacteria found in different sites of a sewage water treatment plant, in duckweed, and in control ponds
| Amt of Aeromonas (CFU/ml) | No. (%) of samples froma:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sewage water
|
Duckweed lagoon
|
Fish culture pond
|
Control (water and sediment) | ||||||
| Water | Sediment | Water | Sediment | Duckweed | Water | Sediment | Duckweed | ||
| ≤102 | 5 (50) | 5 (42) | 1 (9) | 26 (96) | |||||
| 103 | 10 (56) | 3 (37) | 4 (40) | 6 (75) | 4 (33) | 2 (18) | 1 (4) | ||
| 104 | 8 (44) | 1 (13) | 1 (10) | 2 (25) | 3 (25) | 5 (45) | |||
| 105 | 2 (25) | 2 (17) | 3 (27) | 2 (29) | |||||
| ≥106 | 2 (25) | 10 (83) | 5 (71) | ||||||
Number (%) of samples containing specified level of Aeromonas. The total number of samples collected from each site was as follows. Sewage water: for water, n = 18; for sediment, n = 8. Duckweed lagoon: for water, n = 10; for sediment, n = 8; for duckweed, n = 12. Fish culture pond: for water, n = 12; for sediment, n = 11; for duckweed, n = 7. For the control (water and sediment), n = 27.
Table 2 shows the number distribution (CFU/ml or CFU/g) of Aeromonas in the sewage water treatment plant and in the control ponds. Of the 86 samples collected from the sewage water treatment plant, only 11 samples contained less than 102 CFU/ml or CFU/g and the remaining 75 samples contained from 102 to ≥106 CFU/ml or CFU/g. Half of the sediment samples from the sewage water contained more than 105 CFU/g, while the water-phase material contained only 103 to 104 CFU/ml. Of special interest was the fact that in the lagoon, as well as in the fish pond, the duckweed itself appeared to contain significantly higher numbers (>105 CFU/g) of Aeromonas bacteria than the water and sediment (P < 0.001) samples.
Diversities of Aeromonas in different samples.
The diversities of the Aeromonas populations in different sampling sites based on PhP fingerprinting are shown in Table 1. The greatest diversity among the studied Aeromonas populations was found in the control ponds (Di = 0.97), and the lowest diversity was found in human feces from diarrheal patients (Di = 0.83). From the sites sampled at the sewage water treatment plant, the highest Di value was found in the sewage water (Di = 0.96), followed by sediment and duckweed in each sampling site, i.e., in the duckweed lagoon and in the fish pond. No significant difference in diversity was found among the samples, except for the child diarrheal samples (P < 0.001), which showed low diversity.
Major PhP types in different samples.
In total, 1,315 presumed Aeromonas isolates were subjected to typing with the PhP-AE system, and the isolates were found to be distributed over a large number of common (192) and single PhP types, yielding a total diversity index of 0.98 (data not shown). PhP types that included at least 20 isolates and had been isolated on at least 3 different sampling occasions and/or at 3 different sampling sites (not including samples from humans) were referred to as major types (MTs) and arbitrarily designated MT-A to MT-R. In total, 18 such PhP MTs, representing 841 of the 1,315 studied Aeromonas isolates (64%), were found. The remaining PhP types occurred only sporadically and were not further analyzed in this study.
Selection of isolates for further characterization.
The PhP-AE typing is a rapid screening method that can be used to select isolates of interest for further characterization. For the present study, we wanted to investigate whether isolates from different sampling sites or from different sampling occasions belonged to the same clonal group. Several isolates belonging to each MT were selected for further characterization, yielding a total of 90 isolates (at least 4 from each of the 18 MTs representing different sampling sites or sampling occasions). These 90 isolates were further analyzed with the more discriminatory PhP-48 biochemical fingerprinting system. PhP-48 typing could discriminate among a few of the isolates that appeared identical according to PhP-AE typing, and the Di value rose to 0.941 (compared to 0.924 with the PhP-AE system). However, generally the PhP-AE method could reveal the diversity among the various Aeromonas populations in a representative manner, and both systems correlated well. For this reason, discussions on the MTs in the present study are based mainly on PhP-AE data.
Species distribution of major PhP types.
The 90 isolates were first identified at the DNA hybridization group (HG) level by FAME analysis. The FAME analysis allowed us to identify 78% of the isolates at the species level after comparison with the AER48C FAME database, but 20 isolates remained unidentified at the species level. The identified isolates belonged to Aeromonas hydrophila HG1 (n = 25), Aeromonas veronii HG8/10 (n = 30), and Aeromonas caviae HG4 (n = 15). Isolates that could not be identified by FAME were further confirmed by AFLP fingerprinting.
Occurrence of major PhP types in different sampling sites.
The properties of PhP MTs occurring in different sampling sites are presented in Table 3. One PhP type was present in diarrheal samples only, some other types only in the treatment plant, and other types were present in the control ponds, in different parts of the sewage treatment plant, and in healthy humans. Types such as MT-C and MT-G were detected in the samples from diarrheal children, in several sites of the treatment plant, and in the fish samples. Type MT-A (A. hydrophila HG1) was detected in several diarrheal infants but never in the other samples. MT-B and MT-F were found in all sampled sites of the sewage water treatment plant (i.e., sewage water, lagoon, fish pond, and fish), as well as in the control pond that was not related to the treatment plant. Those phenotypes may thus be considered endemic in the sampled area and will be referred to this study as “environmental phenotypes.” Type MT-B was also found in samples from healthy humans. Thus, environmental phenotypes of Aeromonas were recovered from the sewage treatment system, as well as from healthy human feces and the control ponds. Whether those types represent clonal groups that are endemic in the region or whether they have entered the treatment system via, e.g., human feces before this study was set up remains to be clarified. Some types that were found exclusively at several sites in the treatment plant but not in the control ponds may represent persistent strains confined to the sewage water treatment plant.
TABLE 3.
Species and distribution of PhP MTs found in different sampling sites
| Species and PhP MT (na) | Species and biovar or HG identified by:
|
Distribution | |
|---|---|---|---|
| PhP typing | FAME and AFLPb | ||
| A. hydrophila | |||
| A (19) | Unknown | A. hydrophila, HG1 | Child diarrhea |
| B (145) | A. hydrophila | A. hydrophila, HG1 | Human, sewage, lagoon, fish pond, fish, control |
| C (107) | A. hydrophila | A. hydrophila, HG1 | Child diarrhea, sewage, lagoon, fish pond, fish |
| D (23) | A. hydrophila | A. hydrophila, HG1 | Sewage, lagoon, fish pond |
| E (21) | A. hydrophila | A. hydrophila, HG1 | Human, fish pond, control |
| A. veronii | |||
| F (45) | A. veronii biovar sobria | A. veronii, HG8/10 | Sewage, lagoon, fishpond, fish, control |
| G (131) | Unknown | A. veronii, HG8/10 | Child diarrhea, sewage, lagoon, fish pond, fish |
| H (46) | A. veronii biovar sobria | A. veronii, HG8/10 | Sewage, fish pond, fish |
| I (21) | A. veronii biovar sobria | A. veronii, HG8/10 | Lagoon, fish pond, fish |
| J (25) | A. veronii biovar sobria | A. veronii, HG8/10 | Human, sewage, fish pond, fish |
| K (33) | A. veronii biovar sobria | A. veronii, HG8/10 | Human, lagoon, fish pond |
| L (26) | A. veronii biovar sobria | A. veronii, HG8/10 | Lagoon, fish pond, fish |
| M (23) | A. veronii biovar sobria | A. veronii, HG8/10 | Sewage, lagoon, fish pond |
| N (22) | A. veronii biovar sobria | A. veronii, HG8/10 | Fish pond, fish, control |
| O (29) | A. veronii biovar sobria | A. veronii, HG8/10 | Sewage, fish pond, control |
| A. caviae | |||
| P (59) | A. caviae, HG5A | A. caviae, HG4 | Human, sewage, lagoon, fish pond, control |
| Q (29) | A. caviae, HG5A | A. caviae, HG4-like | Child diarrhea, sewage, lagoon, fish |
| R (37) | A. caviae, HG5A | A. caviae, HG4 | Human, sewage, fish |
n, no. of isolates.
Species obtained by comparing AFLP profiles with the AEROLIB database.
Genotyping and phenotyping of major PhP types.
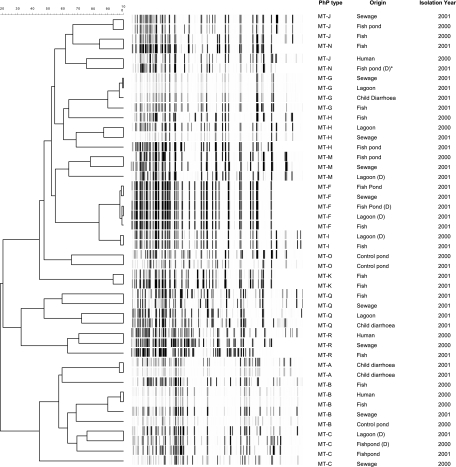
In order to verify the genetic relatedness of major PhP types found in different parts of the sewage water treatment system, further molecular typing of selected isolates was pursued with the more discriminatory AFLP fingerprinting technique (Fig. 1). Overall, AFLP analysis confirmed the grouping of strains into MTs as defined by PhP typing but was also able to further subdivide some of these phenotypes into multiple genotypes. Highly homologous AFLP fingerprinting was found, e.g., among members of PhP type MT-A that had been isolated from two different patients. Among members of PhP type MT-B, some of the isolates could be distinguished by AFLP fingerprinting, whereas some isolates showed highly similar but not identical AFLP fingerprints. Interestingly, isolates belonging to PhP types MT-G and MT-F, which had been isolated in different parts of the sewage treatment plant, all showed same AFLP fingerprints. Furthermore, isolates belonging to PhP types MT-I and MT-J also showed highly homologous AFLP fingerprints (95% to 98%), although they had been isolated during different sampling years. These findings support the hypothesis that phenotypically and genotypically stable clones of Aeromonas could persist in the treatment system over long periods of time (e.g., MT-I and MT-J) and may have spread from hospitalized children suffering from diarrhea to fish produced for human consumption (e.g., MT-G) through the sewage water treatment system.
FIG. 1.
Persistence and transmission of Aeromonas. Unweighted pair group method with arithmetic means/Pearson correlation clustering of digitized and normalized AFLP patterns of a selection of Aeromonas isolates belonging to the major PhP types is shown. *, D indicates duckweed, e.g., isolates associated with duckweed in the fish pond or isolates associated with duckweed in the lagoon.
Distribution of virulence factors among the major PhP types.
Screening of hemolysin and cytotoxin activity of 90 isolates indicated that 39 (43%) isolates showed hemolysin and cytotoxin (Table 4). Furthermore, a cytolytic enterotoxin gene (AHCYTOEN) in A. hydrophila has been reported as a multivirulence gene involved in lethality in mice and in production of hemolysins, cytotoxins, and enterotoxins (8). The AHCYTOEN gene was detected in only 26 (29%) of the investigated strains (Table 4).
TABLE 4.
Frequencies of putative virulence factors among different species of Aeromonas
| Species, HG (na) | No. (%) of isolates positive for:
|
||
|---|---|---|---|
| Hemolysin | Cytotoxin | AHCYTOEN geneb | |
| A. hydrophila, HG1 (25) | 20 (78) | 20 (78) | 16 (70) |
| A. caviae, HG4 (15) | 0 (0) | 0 (0) | 0 (0) |
| A. veronii, HG8/10 (50) | 19 (42) | 19 (38) | 10 (20) |
| Total (90) | 39 (43) | 39 (43) | 26 (29) |
n, no. of isolates tested.
Cytolytic enterotoxin gene.
The frequency of virulence factors among the MT types is shown in Table 5. Within 18 PhP MT types, 9 types were shown to produce virulence factors, whereas the remaining 9 types were negative for all tested virulence factors. Interestingly, the mean cytotoxic titers of the MT-A and MT-G types were significantly higher than those for the other types (P < 0.05). All the tested isolates of these two types were also positive for the cytolytic enterotoxin gene, and both of them were associated with child diarrhea. Furthermore, type MT-A was isolated only from children suffering with diarrhea, whereas type MT-G was isolated not only from children suffering from diarrhea but also from all different sites of the treatment plant.
TABLE 5.
Frequencies of putative virulence factors among the major PhP types of Aeromonas
| Species and PhP MT | % of samples positive, titer (mean ± SE) of:
|
% of samples positive for toxin geneb | |
|---|---|---|---|
| Hemolysin | Cytotoxin | ||
| A. hydrophila | |||
| A | 100, 204.8 ± 31.4 | 100, 819.2 ± 125.4a | 100 |
| B | 80, 4.4 ± 1.6 | 80, 24 ± 11.3 | 60 |
| C | 80, 31.2 ± 24.3 | 80, 217.6 ± 201.7 | 80 |
| D | 80, 23.2 ± 11.6 | 80, 104 ± 47.6 | 60 |
| E | 60, 2.8 ± 1.5 | 60, 9.6 ± 5.8 | 20 |
| A. veronii biovar sobria | |||
| F | 40, 9.6 ± 6.4 | 40, 51.2 ± 31.4 | 40 |
| G | 100, 115.2 ± 37.3 | 100, 614.4 ± 173.6a | 100 |
| H | 60, 6.4 ± 2.9 | 60, 22.4 ± 11.9 | 0 |
| I | 0 | 0 | 0 |
| J | 40, 19.2 ± 12.8 | 40, 102.4 ± 62.7 | 40 |
| K | 20, 3.2 ± 3.2 | 20, 6.4 ± 6.4 | 0 |
| L | 20, 1.6 ± 1.6 | 20, 3.2 ± 3.2 | 0 |
| M | 80, 8.4 ± 3.8 | 80, 46.4 ± 23.2 | 0 |
| N | 0 | 0 | 0 |
| O | 20, 6.4 ± 5.73 | 20, 25.6 ± 25.6 | 20 |
| A. caviae | |||
| P | 0 | 0 | 0 |
| Q | 0 | 0 | 0 |
| R | 0 | 0 | 0 |
P was <0.001 compared to the mean cytotoxic titer for the other groups.
Cytolytic enterotoxin gene.
DISCUSSION
Proper management and/or treatment of sewage water are important environmental issues in many developing countries (6). Efficient removal of pathogens from sewage water is a critical task, since sewage water discharges may increase pathogen contamination in surface water and result in the increase of foodborne and waterborne infections. Generally, sewage water treatment plants reduce the numbers of microorganisms, but the sewage water effluents may still contain high numbers of fecal microorganisms (29, 50). The removal efficiencies for pathogenic and indicator organisms may vary according to the treatment process type and may even vary between organisms (30). The results of the present study indicate that genetically stable pathogenic Aeromonas strains may persist in the duckweed aquaculture-based hospital sewage water treatment plant and that they may be transmitted to fish cultivated for human consumption through the recycling of sewage water.
Whereas it has been shown that Aeromonas is able to persist in drinking water for a prolonged period (33), there is a lack of information available on the survival, persistence, and possible health risks associated with the presence of Aeromonas in sewage and aquaculture systems. To be able to follow the fate of individual strains or clonal groups within a given bacterial population, a sufficiently high number of samples and isolates must be characterized. However, accurate assessment of genetic diversity to address questions regarding genetic relatedness among individuals is not straightforward. For this purpose, a series of techniques has been developed, each exhibiting its own strengths and weaknesses (38). In this investigation, the PhP-AE rapid screening system was used as a first screening method for phenotyping of a high number of isolates. To identify the genetic relatedness within these clonal groups, representative isolates from each clonal group were subjected to further typing and species identification using the more discriminatory PhP-48 system, conventional biochemical tests (when necessary), FAME analysis, and AFLP fingerprinting. In this study, 192 common Aeromonas PhP types were identified from a collection of 1,315 strains isolated from different samples, but only 18 major types were recovered at several occasions and/or at several sites. This suggests that only members of these major types were able to persist during different treatment steps. Furthermore, the finding that strains of types MT-J and MT-I, isolated during two subsequent years in the same sewage treatment plant, displayed highly related AFLP fingerprints supports the hypothesis that phenotypically and genetically stable Aeromonas strains may survive in this type of sewage system for a prolonged period of time.
The transmission of pathogenic bacteria, such as E. coli, Salmonella spp., and Vibrio spp., from the environment to humans via the food chain, e.g., by ingestion of contaminated and/or unprepared foods, such as poultry, beef, pork, eggs, or milk, has been of great concern (4, 43). In this study, Aeromonas strains were isolated from all sewage water treatment plant samples (Table 1), which confirms the ubiquity of these organisms in sewage (3). Interestingly, Aeromonas numbers from the duckweed samples were significantly higher than those from water and sediment of the lagoon and fish ponds (P < 0.001). In contrast, a recent study by Islam and colleagues did not indicate a significant difference in fecal coliform numbers for duckweed samples compared to those for fish culture ponds or to those for the duckweed lagoon from the same sampling source (22). It has previously been reported that aquatic animals and plants may function as reservoirs of pathogenic bacteria and that organisms such as Vibrio cholerae may survive longer on duckweed than in the water on which duckweed was floating or in the control water (19-21). Previously, Parveen and colleagues also observed high Aeromonas numbers on aquatic plants and phytoplankton in Bangladesh (41). In the sewage treatment system studied here, the duckweed grown in sewage water is used as fish feed. Fish may become colonized with high numbers of Aeromonas and provide a reservoir for further transmission (44), which could represent an emerging disadvantage of duckweed-based sewage treatment plants. In fact, we were able to isolate specific Aeromonas clones from both duckweed and fish (MT-F in Fig. 1), which supports the hypothesis that these organisms can use duckweed as transmission vehicles. Such transmission (as far as from the Philippines to California), where bacteria are using aquatic weeds, such as seaweed, as vehicles, has previously been reported for Vibrio cholerae (49). Even transmission of bacteria (Mycobacterium ulcerans) from the environment to humans via fish has been demonstrated in the areas where it is endemic (11). In the present study, the finding of AFLP fingerprints that were indistinguishable between certain Aeromonas strains indicates that phenotypically and genotypically stable clonal lineages of Aeromonas may have the potential to be transmitted from hospitalized children suffering from diarrhea to fish intended for human consumption through the sewage water treatment plant.
Hemolysin and cytotoxin production in Aeromonas strains has often been associated with human gastroenteritis and with soft-tissue infection in fish (42, 47). In the present study, 43% of tested isolates were positive for hemolysin and cytotoxin production. In different studies, Aeromonas cytolytic enterotoxin has been shown to cause activation of proinflammatory cytokines and eicosanoid cascades in macrophages, elevation of cyclic AMP in CHO cell lines and intestinal epithelial cell lines, tissue damage, and fluid secretion response (7-9). The multivirulence gene AHCYTOEN, which encodes this cytolytic enterotoxin, was detected in 29% of the tested isolates. It is noteworthy that this and/or related toxin genes have also previously been found to occur in isolates from the environment and from healthy human control subjects (2). Because of the ubiquitous nature of Aeromonas in food and water, the question again arises of whether Aeromonas bacteria are passengers in the intestinal tract or are primary pathogens. Previously it was shown that a large proportion of Aeromonas isolates suspected to cause diarrhea in patients in Bangladesh belonged to one clonal group, which was later assigned to a new subspecies, Aeromonas hydrophila subsp. dhakensis (16, 32). Furthermore, Wang and colleagues characterized a collection of 121 clinical Aeromonas isolates on the basis of genotypes and cytotoxin titer and demonstrated that high-cytotoxic-titer isolates belonged to one specific genotype (46). In this study, only 2 of 18 major PhP phenotypes displayed significantly higher cytotoxin titers than others, and all the tested isolates from these phenotypes were shown to be positive for the cytolytic enterotoxin gene. Both of them were associated with child diarrhea and DNA fingerprinting by AFLP analysis, suggesting that they are members of the same clonal lineage. Whether this strain caused diarrhea in the patients from whom it was isolated or if these organisms were responsible for diarrhea remains an open question. Altogether, our data suggest that certain clonal lineages of Aeromonas with virulence activities have the potential to spread from hospitalized diarrheal children to the sewage water treatment system and to fish produced for human consumption.
In conclusion, our data indicate that duckweed may serve as a reservoir for further transmission of Aeromonas in aquaculture-based sewage water treatment plants. Furthermore, several phenotypically and genetically stable clonal lineage types of Aeromonas could persist in the sewage treatment plant for several years with the potential to be transmitted from sewage water to cultured fish through the reuse of sewage-grown duckweed. The fact that some of the persistent strains were able to produce putative virulence factors may point to a potential public health risk. Although a detailed assessment of the epidemiological relationship between the prevalence of enteric disease and the consumption of fish produced in the sewage treatment system fell outside the scope of the present investigation, this study suggests that it would be important to perform a community-based study on the prevalence of gastroenteritis in relation to consumption of fish associated with sewage water treatment.
Acknowledgments
This work was supported by SIDA/SAREC grant 1999-255 for research fellowships for Mokhlasur Rahman and the Karolinska Institutet fund. G.H. is a postdoctoral fellow of the Fund for Scientific Research—Flanders (Belgium) (F.W.O.-Vlaanderen).
We thank G. B. Nair at the International Centre for Diarrheal Disease Research in Bangladesh for laboratory support and the Prism Bangladesh Ltd. staff and Kumudini Hospital staff for sampling support. Margo Cnockaert and Marjan De Wachter are acknowledged for excellent technical assistance in identification and typing.
Footnotes
Published ahead of print on 28 December 2006.
REFERENCES
- 1.Alaerts, G. J., M. R. Mahbubar, and P. Kelderman. 1996. Performance analysis of a full-scale duckweed-covered sewage lagoon. Water Res. 30:843-852. [Google Scholar]
- 2.Albert, M. J., M. Ansaruzzaman, K. A. Talukder, A. K. Chopra, I. Kuhn, M. Rahman, A. S. Faruque, M. S. Islam, R. B. Sack, and R. Mollby. 2000. Prevalence of enterotoxin genes in Aeromonas spp. isolated from children with diarrhea, healthy controls, and the environment. J. Clin. Microbiol. 38:3785-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo, R. M., R. M. Arribas, and R. Pares. 1991. Distribution of Aeromonas species in waters with different levels of pollution. J. Appl. Bacteriol. 71:182-186. [DOI] [PubMed] [Google Scholar]
- 4.Brands, D. A., A. E. Inman, C. P. Gerba, C. J. Mare, S. J. Billington, L. A. Saif, J. F. Levine, and L. A. Joens. 2005. Prevalence of Salmonella spp. in oysters in the United States. Appl. Environ. Microbiol. 71:893-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukhari, Z., H. V. Smith, N. Sykes, S. W. Humphreys, C. A. Paton, R. W. A. Girdwood, and C. R. Fricker. 1997. Occurrence of Cryptosporidium spp. oocysts and Giardia spp. cysts in sewage influents and effluents from treatment plants in England. Water Sci. Technol. 35:385-390. [Google Scholar]
- 6.Carr, R. M., U. J. Blumenthal, and D. D. Mara. 2004. Guidelines for the safe use of wastewater in agriculture: revisiting WHO guidelines. Water Sci. Technol. 50:31-38. [PubMed] [Google Scholar]
- 7.Chopra, A. K., and C. W. Houston. 1989. Purification and partial characterization of a cytotonic enterotoxin produced by Aeromonas hydrophila. Can. J. Microbiol. 35:719-727. [DOI] [PubMed] [Google Scholar]
- 8.Chopra, A. K., C. W. Houston, J. W. Peterson, and G. F. Jin. 1993. Cloning, expression, and sequence analysis of a cytolytic enterotoxin gene from Aeromonas hydrophila. Can. J. Microbiol. 39:513-523. [DOI] [PubMed] [Google Scholar]
- 9.Chopra, A. K., X. Xu, D. Ribardo, M. Gonzalez, K. Kuhl, J. W. Peterson, and C. W. Houston. 2000. The cytotoxic enterotoxin of Aeromonas hydrophila induces proinflammatory cytokine production and activates arachidonic acid metabolism in macrophages. Infect. Immun. 68:2808-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, S. C., R. D. Haigh, P. P. Freestone, and P. H. Williams. 2003. Virulence of enteropathogenic Escherichia coli, a global pathogen. Clin. Microbiol. Rev. 16:365-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eddyani, M., D. Ofori-Adjei, G. Teugels, D. De Weirdt, D. Boakye, W. M. Meyers, and F. Portaels. 2004. Potential role for fish in transmission of Mycobacterium ulcerans disease (Buruli ulcer): an environmental study. Appl. Environ. Microbiol. 70:5679-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentry, M. K., and J. M. Dalrymple. 1980. Quantitative microtiter cytotoxicity assay for Shigella toxin. J. Clin. Microbiol. 12:361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haustein, A. T., W. R. Gilman, and P. W. Skillicorn. 1990. Duckweed, a useful strategy for feeding chickens in Third World countries: performance of layers fed with sewage-grown Lemnaceae. Poultry Sci. 69:1835-1844. [Google Scholar]
- 14.Havelaar, A. H., F. M. Schets, A. van Silfhout, W. H. Jansen, G. Wieten, and D. van der Kooij. 1992. Typing of Aeromonas strains from patients with diarrhoea and from drinking water. J. Appl. Bacteriol. 72:435-444. [DOI] [PubMed] [Google Scholar]
- 15.Huys, G., R. Coopman, P. Janssen, and K. Kersters. 1996. High resolution genotypic analysis of the genus Aeromonas by AFLP fingerprinting. Int. J. Syst. Bacteriol. 46:572-580. [DOI] [PubMed] [Google Scholar]
- 16.Huys, G., P. Kampfer, M. J. Albert, I. Kuhn, R. Denys, and J. Swings. 2002. Aeromonas hydrophila subsp. dhakensis subsp. nov., isolated from children with diarrhoea in Bangladesh, and extended description of Aeromonas hydrophila subsp. hydrophila (Chester 1901) Stanier 1943 (approved lists 1980). Int. J. Syst. Evol. Microbiol. 52:705-712. [DOI] [PubMed] [Google Scholar]
- 17.Huys, G., M. Avncanneyt, R. Coopman, P. Jansson, E. Falsen, M. Altwegg, and K. Kersters. 1994. Cellular fatty acid composition as a chemotaxonomic marker for the identification of phenospecies and the hybridization groups in the genus Aeromonas. Int. J. Syst. Bacteriol. 44:651-658. [Google Scholar]
- 18.Huys, G., and J. Swings. 1999. Evaluation of a fluorescent amplified fragment length polymorphism (FAFLP) methodology for the genotypic discrimination of Aeromonas taxa. FEMS Microbiol. Lett. 177:83-92. [Google Scholar]
- 19.Islam, M. S., B. S. Drasar, and D. J. Bradley. 1989. Attachment of toxigenic Vibrio cholerae 01 to various freshwater plants and survival with a filamentous green alga, Rhizoclonium fontanum. J. Trop. Med. Hyg. 92:396-401. [PubMed] [Google Scholar]
- 20.Islam, M. S., B. S. Drasar, and D. J. Bradley. 1990. Survival of toxigenic Vibrio cholerae O1 with a common duckweed, Lemna minor, in artificial aquatic ecosystems. Trans. R. Soc. Trop. Med. Hyg. 84:422-424. [DOI] [PubMed] [Google Scholar]
- 21.Islam, M. S., B. S. Drasar, and R. B. Sack. 1994. The aquatic flora and fauna as reservoirs of Vibrio cholerae: a review. J. Diarrhoeal Dis. Res. 12:87-96. [PubMed] [Google Scholar]
- 22.Islam, M. S., M. S. Kabir, S. I. Khan, M. Ekramullah, G. B. Nair, R. B. Sack, and D. A. Sack. 2004. Wastewater-grown duckweed may be safely used as fish feed. Can. J. Microbiol. 50:51-56. [DOI] [PubMed] [Google Scholar]
- 23.Iversen, A., I. Kuhn, M. Rahman, A. Franklin, L. G. Burman, B. Olsson-Liljequist, E. Torell, and R. Mollby. 2004. Evidence for transmission between humans and the environment of a nosocomial strain of Enterococcus faecium. Environ. Microbiol. 6:55-59. [DOI] [PubMed] [Google Scholar]
- 24.Janda, J. M., and S. L. Abbott. 1998. Evolving concepts regarding the genus Aeromonas: an expanding panorama of species, disease presentations, and unanswered questions. Clin. Infect. Dis. 27:332-344. [DOI] [PubMed] [Google Scholar]
- 25.Kahana, Y., and A. Tal. 2000. Technological solutions in support of sustainable use of water in metropolitan areas. Interdisciplinary Center for Technological Analysis and Forecasting, Tel Aviv University, Tel Aviv, Israel.
- 26.Kanclerski, K., and R. Mollby. 1987. A simple and exact two-point interpolation method for determination of haemolytic activity in microtiter plates. Acta Pathol. Microbiol. Immunol. Scand. Sect. B 95:175-179. [DOI] [PubMed] [Google Scholar]
- 27.Karl, T. R., R. W. Knight, and N. Plummer. 1995. Trends in high-frequency climate variability in the twentieth century. Nature 377:217-220. [Google Scholar]
- 28.Keawvichit, R., K. Wongworapat, P. Putsyainant, A. Silprasert, and S. Karnchanawong. 2001. Parasitic and bacterial contamination in collards using effluent from treated domestic wastewater in Chiang Mai, Thailand. Southeast Asian J. Trop. Med. Public Health 32(Suppl. 2):240-244. [PubMed] [Google Scholar]
- 29.Koivunen, J., E. Lanki, R. L. Rajala, A. Siitonen, and H. Heinonen-Tanski. 2001. Determination of salmonellae from municipal wastewaters. Water Sci. Technol. 43:221-224. [PubMed] [Google Scholar]
- 30.Koivunen, J., A. Siitonen, and H. Heinonen-Tanski. 2003. Elimination of enteric bacteria in biological-chemical wastewater treatment and tertiary filtration units. Water Res. 37:690-698. [DOI] [PubMed] [Google Scholar]
- 31.Korner, S., G. B. Lyatuu, and J. E. Vermaat. 1998. The influence of Lemna gibba L. on the degradation of organic material in duckweed-covered domestic wastewater. Water Res. 32:3092-3098. [Google Scholar]
- 32.Kuhn, I., M. J. Albert, M. Ansaruzzaman, N. A. Bhuiyan, S. A. Alabi, M. S. Islam, P. K. Neogi, G. Huys, P. Janssen, K. Kersters, and R. Mollby. 1997. Characterization of Aeromonas spp. isolated from humans with diarrhea, from healthy controls, and from surface water in Bangladesh. J. Clin. Microbiol. 35:369-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhn, I., G. Allestam, G. Huys, P. Janssen, K. Kersters, K. Krovacek, and T. X. Stenstrom. 1997. Diversity, persistence and virulence of Aeromonas strains isolated from drinking water distribution systems in Sweden. Appl. Environ. Microbiol. 63:2708-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhn, I., G. Huys, R. Coopman, K. Kersters, and P. Janssen. 1997. A 4-year study of the diversity and persistence of coliforms and Aeromonas in the water of a Swedish drinking water well. Can. J. Microbiol. 43:9-16. [DOI] [PubMed] [Google Scholar]
- 35.Leng, R. A., J. H. Stambolie, and R. Bell. October. 1995. Duckweed—a potential high-protein feed resource for domestic animals and fish. Livestock Res. Rural Dev. 7. http://www.cipav.org.co/lrrd/.
- 36.McMahon, M. A., and I. G. Wilson. 2001. The occurrence of enteric pathogens and Aeromonas species in organic vegetables. Int. J. Food Microbiol. 70:155-162. [DOI] [PubMed] [Google Scholar]
- 37.Messi, P., E. Guerrieri, and M. Bondi. 2002. Survival of an Aeromonas hydrophila in an artificial mineral water microcosm. Water Res. 36:3410-3415. [DOI] [PubMed] [Google Scholar]
- 38.Mueller, U. G., and L. L. Wolfenbarger. 1999. AFLP genotyping and fingerprinting. Trends Ecol. Evol. 14:389-394. [DOI] [PubMed] [Google Scholar]
- 39.Möllby, R., I. Kühn, and M. Katouli. 1993. Computerized biochemical fingerprinting. A new tool for typing of bacteria. Rev. Med. Microbiol. 4:231-241. [Google Scholar]
- 40.Oppegaard, H., T. M. Steinum, and Y. Wasteson. 2001. Horizontal transfer of a multi-drug resistance plasmid between coliform bacteria of human and bovine origin in a farm environment. Appl. Environ. Microbiol. 67:3732-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parveen, S., M. S. Islam, and A. Huq. 1995. Abundance of Aeromonas spp. in river and lake waters in and around Dhaka, Bangladesh. J. Diarrhoeal Dis. Res. 13:183-186. [PubMed] [Google Scholar]
- 42.Rahman, M., P. Colque-Navarro, I. Kuhn, G. Huys, J. Swings, and R. Mollby. 2002. Identification and characterization of pathogenic Aeromonas veronii biovar sobria associated with epizootic ulcerative syndrome in fish in Bangladesh. Appl. Environ. Microbiol. 68:650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rangel, J. M., P. H. Sparling, C. Crowe, P. M. Griffin, and D. L. Swerdlow. 2005. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg. Infect. Dis. 11:603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rawls, J. F., B. S. Samuel, and J. I. Gordon. 2004. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Natl. Acad. Sci. USA 101:4596-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sneath, P. H. A., and R. R. Sokal. 1973. Numerical taxonomy. W. H. Freeman and Co., New York, NY.
- 46.Wang, G., C. G. Clark, C. Liu, C. Pucknell, C. K. Munro, T. M. Kruk, R. Caldeira, D. L. Woodward, and F. G. Rodgers. 2003. Detection and characterization of the hemolysin genes in Aeromonas hydrophila and Aeromonas sobria by multiplex PCR. J. Clin. Microbiol. 41:1048-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, G., K. D. Tyler, C. K. Munro, and W. M. Johnson. 1996. Characterization of cytotoxic, hemolytic Aeromonas caviae clinical isolates and their identification by determining presence of a unique hemolysin gene. J. Clin. Microbiol. 34:3203-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winfield, M. D., and E. A. Groisman. 2003. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microbiol. 69:3687-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vugia, D. J., A. M. Shefer, J. Douglas, K. D. Greene, R. G. Bryant, and S. B. Werner. 1997. Cholera from raw seaweed transported from the Philippines to California. J. Clin. Microbiol. 35:284-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yaziz, M. I., and B. J. Lloyd. 1982. The removal of Salmonella enteritidis in activated sludge. J. Appl. Bacteriol. 53:169-172. [DOI] [PubMed] [Google Scholar]
- 51.Zirschky, J., and S. C. Reed. 1988. The use of duckweed for wastewater treatment. J. Water Pollut. Control Fed. 60:1253-1258. [Google Scholar]