Abstract
Currently, diagnosis of acute hepatitis E virus (HEV) in patients is primarily based on anti-HEV immunoglobulin M (IgM) detection. However, several investigations suggest the use of HEV-specific IgA for diagnosing acute HEV infections. We evaluated two commercially available assays, an IgA enzyme-linked immunosorbent assay (ELISA) (Diacheck) and an adapted immunoblot protocol (Mikrogen) for IgA detection and compared the performance in genotype 1- and 3-infected patients. The specificity of the IgA assays was high, with no positive reactions in a control group of 18 acute hepatitis patients who were negative for HEV. The sensitivity calculated in nine PCR-positive type 1-infected patients was 100% in both assays but was clearly lower in genotype 3-infected patients (n = 14), with sensitivities of only 67% and 57% for the ELISA and immunoblot assay, respectively. The lower IgA responses detected in genotype 3-infected patients could be caused by the use of only the genotype 1 and 2 antigens in the serological assays. Interestingly in two patients with possible infection through blood transfusion no response or intermediate IgA responses were detected, and this might confirm the parenteral route of transmission. In both the type 1- and type 3-infected patients both the IgA and IgM responses disappeared simultaneously. We conclude that IgA detection is of limited value for the serodiagnosis of acute HEV cases, particularly with genotype 3.
Hepatitis E virus (HEV) infections are recognized in The Netherlands as an imported disease related to travel to regions where HEV is endemic, but the disease also results from indigenous transmission of HEV (9, 25). HEV is transmitted primarily by the fecal-oral route, with mucosal replication and shedding of the virus (2, 17), but transmission by blood transfusion has also been described (1, 12, 14, 15, 26). Hepatitis E is caused by viruses belonging to the family Hepeviridae and is typically a self-limiting disease with variable severity, presenting as acute icteric hepatitis with clinical symptoms similar to those of hepatitis A (10). In The Netherlands locally acquired HEV cases are generally caused by genotype 3, and in the travel-related cases genotype 1 is frequently detected (9, 24, 25).
Because viremia is thought to exist only during the acute phase of illness, the diagnosis of an HEV infection is mainly dependent on serology (10). Both HEV-specific immunoglobulin M (IgM) and IgG are generally detectable at the onset of disease, but the titers of IgM decline within 3 months in most patients during early convalescence (5, 8, 13). In patients with clear HEV-specific IgG responses in the absence of IgM, it cannot be concluded with certainty whether the IgG response reflects past or recent contact with HEV since IgG can be detected in most patients for at least 1 year after acute infection (3, 5, 8, 13). HEV-specific IgM is used as a reliable and sensitive marker for recent HEV infection; however, the sensitivity is limited to the acute phase of disease since IgM levels decline rapidly and will be undetectable if samples are collected late after onset of disease.
HEV-specific IgA has been detected in sera from acute-HEV patients, and the presence of HEV-specific IgA in combination with IgM was found to be highly specific for the serodiagnosis of acute HEV infections (4, 11, 16, 19, 21, 22). As the duration of the IgA response seemed limited (19), it was suggested that anti-HEV IgA detection may be useful to discriminate acute and past infections for serological diagnosis of recent (subclinical) HEV infection (15). Before possible application of IgA serology the clinical and epidemiological implication of a positive IgA response needs to be further investigated.
We investigated if detection of IgA responses in hepatitis patients with suspected HEV infection is of additional value to IgM detection for serodiagnosing acute HEV infections. For this purpose we used a commercially available IgA enzyme-linked immunosorbent assay (ELISA) from Diacheck and adapted the IgG/IgM immunoblot assay of Mikrogen for the detection of IgA. We also compared IgA responses in samples from locally acquired genotype 3 HEV infections with unknown mode of transmission to results in travel-related cases (genotype 1 infections).
MATERIALS AND METHODS
Clinical samples.
For evaluating the usefulness of detecting HEV-specific IgA for HEV serology five groups of sera were examined. Group 1 comprised negative-control serum samples from 18 patients with acute hepatitis that were serologically and virologically considered negative for an acute HEV infection (negative for IgM by ELISA and immunoblot assay and a negative PCR result). Most patients in this group were also IgG negative (n = 13), but in five cases low-level IgG responses were detected in the immunoblot assay (score of 4 to 6). Group 2 comprised positive-control sera collected from 23 acute-HEV patients with a positive PCR result for their serum. Nine patients (10 samples) were infected with genotype 1 strains, and 14 patients (15 samples) were infected with a genotype 3 strain. All patients had clinical symptoms of acute hepatitis and/or elevated liver enzymes. These samples were collected during the acute phase (≤3 weeks after onset of disease). Group 3 comprised follow-up samples from 12 patients (14 samples) identified based on positive IgM and IgG responses and/or a positive PCR result in a previous serum sample. These samples were collected between the start and approximately 2 months after onset of disease. Group 4 comprised sera from 20 patients diagnosed with past or recent HEV infection with an IgG response in the immunoblot assay (blot score of 4 to 12) in the absence of a positive IgM response. Group 5 comprised sera from a group of six patients with a positive IgM result in the absence of IgG in which a possible early infection could not be confirmed with a positive PCR result and which were analyzed for the presence of IgA.
IgG/IgM HEV ELISA (Genelabs Diagnostics).
The HEV-specific IgG and IgM ELISAs were performed according to the manufacturer's instructions (Genelabs Diagnostics Inc., California). The ELISA is based on recombinant proteins from the ORF2 gene, which encodes the major capsid protein, and the ORF3 gene, which encodes a short protein of unknown function, from genotype 1 and 2 HEV strains expressed in Escherichia coli (Genelabs) (6, 7, 28). Positive- and negative-control samples provided with the kit were included in each run. Cutoff values were calculated as 0.500 (for IgG) or 0.400 (for IgM) plus the mean absorbance of the nonreactive controls. Ratios of ≥1 (optical density [OD] value of the test sample divided by the cutoff) were considered positive.
IgA HEV ELISA (Diacheck).
Prior to testing for HEV-specific IgA antibodies, sera were depleted of IgG with Gullsorb (Gull Laboratories, 's-Hertogenbosch, The Netherlands) to prevent possible interisotype competition. The HEV-specific IgA ELISA was performed according to the manufacturer's instructions (Diacheck, Switzerland). In brief, serum dilutions of 1/21 were added to the plates and plates were incubated at 37°C for 30 min. After a washing, conjugate was added, and plates were incubated for 30 min at 37°C. The plates were washed, and 100 μl per well of tetramethylbenzidine substrate was added. The reaction was stopped after 15 min, and the plates were read at 450 nm (second filter, 630 nm). The cutoff level was defined as the average OD plus 3 standard deviations of results obtained with the negative-control sera. Ratios of ≥1 (OD value of the test sample divided by the cutoff) were considered positive.
IgG/IgM/adapted IgA HEV RecomBlot (Mikrogen).
The HEV immunoblot assay was performed according to the manufacturer's instructions for the detection of IgM and IgG and adapted for the detection of HEV-specific IgA. For this purpose the optimal dilution of 1/500 of the anti-human IgA peroxidase conjugate (Sigma, Missouri) was used. Antigens on the immunoblot are the N-terminal part of the capsid antigen (glutathione S-transferase fusion protein O2N, 50 kDa), the C-terminal part of the capsid antigen (triple band) (O2C, 38 to 41 kDa), the middle part of the capsid antigen (O2M, 28 kDa), and the ORF3 protein (O3, 15 kDa) of genotype 1 and 2 HEV. Prior to testing for HEV-specific IgM and IgA antibodies, sera were depleted of IgG with Gullsorb (Gull Laboratories, 's-Hertogenbosch, The Netherlands) to prevent possible interisotype competition. All four bands on the immunoblot were scored on intensity (score of 0 to 3), with a maximal score of 12. A specimen was considered positive for anti-HEV IgM or IgG when the total score of the test was higher then 5 or 3, respectively. Samples scoring exactly 5 (IgM) or 3 (IgG) were considered intermediate.
RESULTS
HEV-specific IgA ELISA and immunoblot responses in the control group.
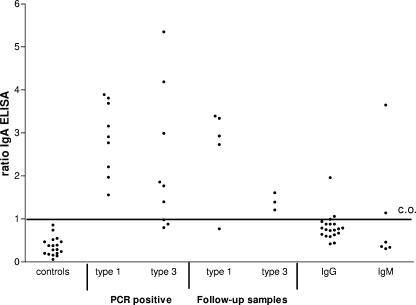
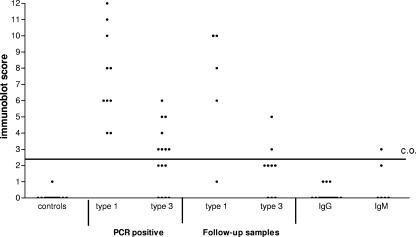
A total of 18 serum samples from the negative-control group were tested to estimate the specificity of the HEV-specific IgA ELISA. The cutoff value was set at the average OD in this control group plus three times the standard deviation. None of the samples in this group had a positive IgA response in the ELISA (Fig. 1). Seventeen samples were also tested in the IgA immunoblot assay (Fig. 2). All controls except one had an immunoblot score of 0. The only response detected in one patient was against the O2M antigen band and was weakly reactive, with a score of only 1. The cutoff level of the IgA-specific immunoblot assay was set at 3, and samples with a score of 2 were considered intermediate. The specificities of both the IgA ELISA and immunoblot assay were 100%.
FIG. 1.
HEV-specific IgA antibodies measured by ELISA in controls and in hepatitis patients. Serum samples were from controls (n = 18), 8 type 1-infected PCR-positive patients (9 samples), 9 type 3-infected PCR-positive patients, 5 type 1 and 3 type 3 follow-up samples from serologically confirmed HEV patients based on a positive IgM and IgG response or PCR-positive result in a previous sample, 20 patients with an IgG response in the immunoblot assay (score of 4 to 12) in the absence of an IgM response, and sera from 6 hepatitis patient with an IgM response only.
FIG. 2.
HEV-specific IgA antibodies measured by immunoblot assay in controls and in hepatitis patients. Serum samples were from controls (n = 17), 9 type 1-infected PCR-positive patients (10 samples), 14 type 3-infected PCR-positive patients (15 samples), 5 type 1 and 8 (9 samples) follow-up samples from serologically confirmed HEV patients based on a positive IgM and IgG response or PCR positive result in a previous sample, 20 patients with IgG response in the immunoblot assay (score of 4 to 12) in the absence of an IgM response, and sera from 6 hepatitis patient with an IgM response only.
HEV-specific IgA ELISA and immunoblot assay responses in type 1-infected and type 3-infected PCR-positive patients.
Sera from eight patients who were infected with type 1 HEV and nine patients with a type 3 infection were analyzed in the IgA ELISA. All of the HEV type 1-infected patients tested positive for IgA (Fig. 1), and six out of the nine (67%) type 3-infected patients were IgA positive in the ELISA. All the type 1-infected patients also tested positive for IgA in the immunoblot assay, with scores of 4 to 12. IgA responses in the type 3-infected patients were clearly lower (range, 0 to 6) than those in the type 1-infected patients (Fig. 2). In total only 57% of the type 3-infected patients (8 out of 14) tested positive for IgA in the immunoblot assay. Three patients were IgA negative, and three patients were considered intermediate. The two patients who had received multiple blood transfusions were both IgA negative in the ELISA, and one patient tested negative and one had an intermediate result in the immunoblot assay.
HEV-specific IgA ELISA and immunoblot responses in serologically confirmed acute-HEV patients.
IgA responses were detected in the group of serologically confirmed HEV patients on the basis of the presence of HEV-specific IgM and IgG antibodies and/or a positive PCR result in a previous sample. These follow-up samples were taken within 2 months after the onset of disease. The latest positive IgA sample was at 22 days postonset, and at approximately 1 month we detected only weak IgA (n = 2) reactions; thereafter, no IgA was detected. Four out of the five (80%) type 1-infected patients were still IgA positive in the ELISA and the immunoblot assay (Fig. 1 and 2). Compared to the type 1-infected group again the responses in the type 3-infected patients were lower on average in both the ELISA and the immunoblot assay. The three tested type 3-infected patients were all positive in the IgA ELISA but had ratios of <2, and only two out of eight type 3-infected patients (25%) were positive for IgA in the immunoblot assay. In general the IgA responses seemed to decline rapidly over time in both the type 1- and 3-infected patients.
HEV-specific IgA ELISA and immunoblot responses in patients with a solitary IgG or IgM response to HEV.
We investigated if detection of HEV-specific IgA was of additional value for patients with solitary IgM (n = 6) or IgG (n = 20) responses to possibly discriminate recent from past infections. In the IgM-positive patients two patients had detectable IgA in the ELISA and only one patient had a positive score of 3 in the IgA immunoblot assay (Fig. 1 and 2). In only 2 out of the 20 patients with solitary IgG responses (10%) was an IgA response detected in the IgA ELISA (ratios of 1.06 and 1.96) (Fig. 1), but none of the patients tested positive in the IgA immunoblot assay (Fig. 2).
Comparison of responses to the individual antigen bands in the immunoblot assay in type 1- and type 3-infected patients.
The IgA reactivities of serum samples from 15 type 1-infected and 24 type 3-infected HEV patients and 17 control patients to individual bands in the immunoblot assay (O2N, O2M, O2C, and O3) were scored separately and compared (Table 1). If only immunoblot scores for an individual band of 2 to 3 were considered reactive, the O2N, O2C, O2M, and O3 antigens did not react with IgA in the control sera (n = 17). The type 1-infected patients had IgA responses to antigens O2N, O2C, O2M, and O3, with reactivity in 87%, 80%, 40%, and 53% of the cases, respectively. The responses were lower in the type 3-infected patients, with only 13%, 33%, 0%, and 25% positives, respectively.
TABLE 1.
IgA responses against the individual antigen bands in the immunoblot assay in the controls and in type 1- and type 3-infected HEV patients
| Group | n | No. (%) of patients with reaction to antigen:
|
|||
|---|---|---|---|---|---|
| O2N | O2C | O2M | O3 | ||
| Controls | 17 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Type 1 infection | 15 | 13 (87) | 12 (80) | 6 (40) | 8 (53) |
| Type 3 infection | 24 | 3 (13) | 8 (33) | 0 (0) | 6 (25) |
DISCUSSION
We investigated if detection of IgA responses in hepatitis patients with suspected HEV infection is of additional value to IgM response detection for serodiagnosing acute HEV infections. In none of the control hepatitis patients (n = 18) was an IgA response against HEV detected by both the IgA ELISA and the adapted IgA immunoblot assay, indicating a high specificity of the assays.
IgA responses were clearly more prominent in the type 1 patients compared to the proven type 3 infections. The sensitivity calculated for nine PCR-positive type 1-infected patients was high in both assays (100%) but was clearly lower (57 to 67%) for type 3-infected patients (n = 14). Also, significantly lower responses to the O2N, O2C, and O2M antigens were detected in the type 3-infected patients than in type 1-infected cases. These differences between type 1- and type 3-infected patients could be explained by the use of the homologous type 1 antigens in the assays. This effect was to a lesser extent also observed for the IgM and IgG responses (9). However, this effect seems more prominent for the IgA response. The lower IgA responses in type 3 patients could also indicate differences in the pathogenic capabilities of type 3 strains versus the type 1 strains. In several countries HEV genotype 3 is found to be closely related to HEV strains detected in domestic pigs and zoonotic food-borne transmission of HEV genotype 3 strains is shown (18, 20, 23, 27). If HEV genotype 3 infections are indeed zoonotic, infections with genotype 3 could cause a milder infection than the human-related genotype 1 strains. Type 1 infection may cause more-extensive mucosal infection, leading to more and higher IgA antibody production and mucosal stimulation than type 3, with limited stimulation of the mucosal immunity.
Transmission of HEV by blood transfusion has been described previously (1, 12, 15, 21, 26), and two patients who received multiple blood transfusions within the known incubation period before onset of disease were included in our study. Interestingly, no response or intermediate IgA responses were detected in these recipients, and this might confirm the possible parenteral route of HEV transmission. Transmission of HEV through blood transfusions evades the mucosal immune system and probably would not lead to extensive viral replication at mucosal sites and therefore would not be able to stimulate an IgA response.
In the patients with clear HEV-specific IgG responses in the absence of IgM, it cannot be concluded with certainty whether the IgG response reflects past or recent contact with HEV. Most HEV patients produced a clear IgA response, but the IgA levels disappeared usually at the same rate as the IgM antibodies. We detected only two IgA-positive cases with the ELISA and none with the immunoblot assay in patients with an IgG response in the absence of IgM. Possibly these IgG- and IgA-positive patients had recent contact with HEV, but the added value of this application seems low (10%). For this reason detection of IgA antibodies has limited additional value over the detection of HEV-specific IgM for serodiagnosing an acute HEV infection. Since IgM is related to recent contact with the pathogen, detection of it is preferable to detection of IgA.
We conclude that in individual cases additional IgA testing might be useful but that for routine HEV serology there is limited additional value for HEV-specific IgA testing, since detection in all acute-HEV patients is readily accomplished with the standard IgG and IgM immunoassays.
Acknowledgments
We thank René Benne and Peter Schneeberger for the collection of sera from HEV patients.
Footnotes
Published ahead of print on 31 January 2007.
REFERENCES
- 1.Arankalle, V. A., and L. P. Chobe. 2000. Retrospective analysis of blood transfusion recipients: evidence for post-transfusion hepatitis E. Vox Sang. 79:72-74. [DOI] [PubMed] [Google Scholar]
- 2.Balayan, M. S., A. G. Andjaparidze, S. S. Savinskaya, E. S. Ketiladze, D. M. Braginsky, A. P. Savinov, and V. F. Poleschuk. 1983. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 20:23-31. [DOI] [PubMed] [Google Scholar]
- 3.Chadha, M. S., A. M. Walimbe, and V. A. Arankalle. 1999. Retrospective serological analysis of hepatitis E patients: a long-term follow-up study. J. Viral Hepat. 6:457-461. [DOI] [PubMed] [Google Scholar]
- 4.Chau, K. H., G. J. Dawson, K. M. Bile, L. O. Magnius, M. H. Sjogren, and I. K. Mushahwar. 1993. Detection of IgA class antibody to hepatitis E virus in serum samples from patients with hepatitis E virus infection. J. Med. Virol. 40:334-338. [DOI] [PubMed] [Google Scholar]
- 5.Chow, W. C., A. S. Lee, G. K. Lim, W. K. Cheong, R. Chong, C. K. Tan, C. K. Yap, C. J. Oon, and H. S. Ng. 1997. Acute viral hepatitis: clinical and serological studies in Singapore. J. Clin. Gastroenterol. 24:235-238. [DOI] [PubMed] [Google Scholar]
- 6.Colak, D., D. Ogunc, F. Gunsteren, S. Velipasaoglu, M. R. Aktekin, and M. Gultekin. 2002. Seroprevalence of antibodies to hepatitis A and E viruses in pediatric age groups in turkey. Acta Microbiol. Immunol. Hung. 49:93-97. [DOI] [PubMed] [Google Scholar]
- 7.Daniel, H. D., A. Warier, P. Abraham, and G. Sridharan. 2004. Age-wise exposure rates to hepatitis E virus in a south Indian patient population without liver disease. Am. J. Trop. Med. Hyg. 71:675-678. [PubMed] [Google Scholar]
- 8.Favorov, M. O., H. A. Fields, M. A. Purdy, T. L. Yashina, A. G. Aleksandrov, M. J. Alter, D. M. Yarasheva, D. W. Bradley, and H. S. Margolis. 1992. Serologic identification of hepatitis E virus infection in epidemic and endemic settings. J. Med. Virol. 36:246-250. [DOI] [PubMed] [Google Scholar]
- 9.Herremans, M., H. Vennema, J. Bakker, B. van der Veer, R. Benne, K. Waar, B. Hendrixks, P. Schneeberger, G. Blaauw, M. Kooiman, and M. P. G. Koopmans. 2007. Swine-like hepatitis E viruses are a cause of unexplained hepatitis in the Netherlands. J. Viral Hepatol 14:140-146. [DOI] [PubMed]
- 10.Jameel, S. 1999. Molecular biology and pathogenesis of hepatitis E virus. Expert Rev. Mol. Med. 1999:1-16. [DOI] [PubMed] [Google Scholar]
- 11.Joshi, M. S., A. M. Walimbe, V. A. Arankalle, M. S. Chadha, and S. D. Chitambar. 2002. Hepatitis E antibody profiles in serum and urine. J. Clin. Lab. Anal. 16:137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khuroo, M. S., S. Kamili, and G. N. Yattoo. 2004. Hepatitis E virus infection may be transmitted through blood transfusion in an endemic area. J. Gastroenterol. Hepatol. 19:778-784. [DOI] [PubMed] [Google Scholar]
- 13.Koshy, A., S. Grover, K. C. Hyams, M. A. Shabrawy, A. Pacsa, B. Al Nakib, S. A. Zaidi, A. A. Al Anezi, S. Al Mufti, J. Burans, M. Carl, and A. L. Richards. 1996. Short term IgM and IgG antibody responses to hepatitis E virus infection. Scand. J. Infect. Dis. 28:439-441. [DOI] [PubMed] [Google Scholar]
- 14.Matsubayashi, K., Y. Nagaoka, H. Sakata, S. Sato, K. Fukai, T. Kato, K. Takahashi, S. Mishiro, M. Imai, N. Takeda, and H. Ikeda. 2004. Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion 44:934-940. [DOI] [PubMed] [Google Scholar]
- 15.Mitsui, T., Y. Tsukamoto, C. Yamazaki, K. Masuko, F. Tsuda, M. Takahashi, T. Nishizawa, and H. Okamoto. 2004. Prevalence of hepatitis E virus infection among hemodialysis patients in Japan: evidence for infection with genotype 3 HEV by blood transfusion. J. Med. Virol. 74:563-572. [DOI] [PubMed] [Google Scholar]
- 16.Mushahwar, I. K., G. J. Dawson, and G. R. Reyes. 1996. Hepatitis E virus: molecular biology and diagnosis Eur. J. Gastroenterol. Hepatol. 8:312-318. [PubMed] [Google Scholar]
- 17.Nanda, S. K., I. H. Ansari, S. K. Acharya, S. Jameel, and S. K. Panda. 1995. Protraced viremia during acute sporadic hepatitis E virus infection. Gastroenterology 108:225-230. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi, K., N. Kitajima, N. Abe, and S. Mishiro. 2004. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology 330:501-505. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi, M., S. Kusakai, H. Mizuo, K. Suzuki, K. Fujimura, K. Masuko, Y. Sugai, T. Aikawa, T. Nishizawa, and H. Okamoto. 2005. Simultaneous detection of immunoglobulin A (IgA) and IgM antibodies against hepatitis E virus (HEV) is highly specific for diagnosis of acute HEV infection. J. Clin. Microbiol. 43:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tei, S., N. Kitajima, K. Takahashi, and S. Mishiro. 2003. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362:371-373. [DOI] [PubMed] [Google Scholar]
- 21.Tian, D. Y., Y. Chen, and N. S. Xia. 2006. Significance of serum IgA in patients with acute hepatitis E virus infection. World J. Gastroenterol. 12:3919-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokita, H., H. Harada, Y. Gotanda, M. Takahashi, T. Nishizawa, and H. Okamoto. 2003. Molecular and serological characterization of sporadic acute hepatitis E in a Japanese patient infected with a genotype III hepatitis virus in 1993. J. Gen. Virol. 84:421-427. [DOI] [PubMed] [Google Scholar]
- 23.van der Poel, W. H. M., F. Verschoor, R. van der Heide, M. Kooreman, and A. M. de Roda Husman. 2001. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg. Infect. Dis. 7:970-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waar, K., M. M. P. T. Herremans, H. Vennema, M. P. G. Koopmans, and C. A. Benne. 2005. Hepatitis E is a cause of unexplained hepatitis in The Netherlands. J. Clin. Virol. 33:145-149. [DOI] [PubMed] [Google Scholar]
- 25.Widdowson, M. A., W. J. M. Jaspers, W. H. M. van der Poel, F. Verschoor, A. M. de Roda Husman, H. L. J. Winter, H. L. Zaaijer, and M. Koopmans. 2003. Cluster of cases of acute hepatitis associated with hepatitis E virus infection acquired in The Netherlands. Clin. Infect. Dis. 36:29-33. [DOI] [PubMed] [Google Scholar]
- 26.Xia, N. S., J. Zhang, Y. J. Zheng, S. X. Ge, X. Z. Ye, and S. H. Ou. 2004. Transfusion of plasma from a blood donor induced hepatitis E in rhesus monkey. Vox Sang. 86:45-47. [DOI] [PubMed] [Google Scholar]
- 27.Yazaki, Y., H. Mizuo, M. Takahashi, T. Nishizawa, N. Sasaki, Y. Gotanda, and H. Okamoto. 2003. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J. Gen. Virol. 84:2351-2357. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, J. Z., S. W. Im, S. H. Lau, T. N. Chau, S. T. Lai, S. P. Ng, M. Peiris, C. Tse, T. K. Ng, and M. H. Ng. 2002. Occurrence of hepatitis E virus IgM, low avidity IgG serum antibodies, and viremia in sporadic cases of non-A, -B, and -C acute hepatitis. J. Med. Virol. 66:40-48. [DOI] [PubMed] [Google Scholar]