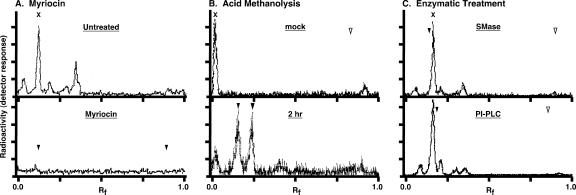
FIG. 2.
Myriocin inhibition of de novo sphingolipid synthesis in trypanosomes. (A) Bloodstream-stage trypanosomes grown for 4 h in the presence (bottom) or absence (top) of 200 nM myriocin were pulse-radiolabeled with 50 μCi/ml of [3H]serine for 4 h. Base-resistant lipids were extracted as described in Materials and Methods and analyzed by TLC. The mobilities of sphingomyelin (Rf, 0.12) and ceramide (Rf, 0.89) standards were determined by iodine staining (arrowheads, bottom panel only). (B) Saponified [3H]serine-labeled lipids were subjected to mock treatment (top) or acid methanolysis (bottom) (2 h) as described in Materials and Methods and then analyzed by TLC. Ceramide was added as an internal marker/substrate prior to hydrolysis. The mobilities of unhydrolyzed ceramide (open arrowhead) (Rf, 0.83), the sphingosine product (shaded arrowhead) (Rf, 0.25), and the sphinganine product (solid arrowhead) (Rf, 0.15) are indicated. (C) Base-resistant [3H]serine-labeled lipids from untreated bloodstream trypanosomes were either mock treated (not shown) or digested with either sphingomyelinase (SMase) (top) or bacterial PI-PLC (bottom) with internal sphingomyelin and PI controls, respectively. Samples were prepared and analyzed as for panel A. Solid arrowheads indicate relative mobilities of internal sphingomyelin (top) (Rf, 0.14) and PI (bottom) (Rf, 0.22) control substrates (from the mock treatments), and open arrowheads indicate the respective ceramide (top) (Rf, 0.9) and diacylglycerol (bottom) (Rf, 0.8) products. Note that the solvent systems used for panels A and C are different from that for panel B.