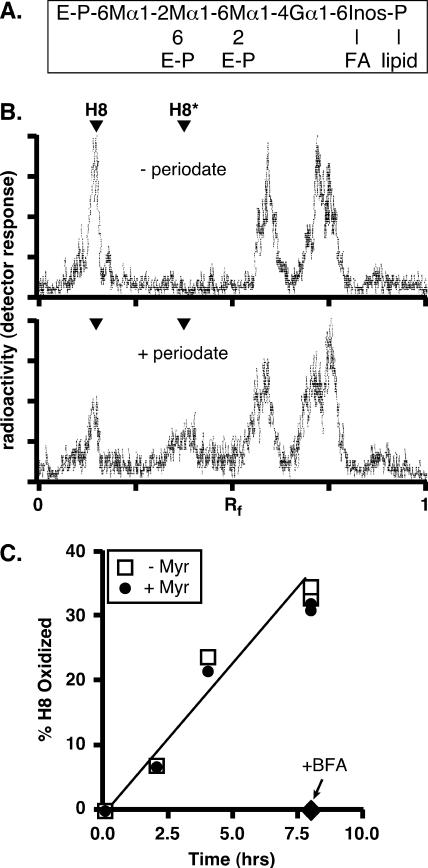
FIG. 8.
Transport of free GPIs to the cell surface in HeLa cells is unaffected by myriocin. (A) Structure of the mammalian GPI H8, showing glycosidic linkages and sites of ethanolamine-phosphate (E-P) attachment. M, mannose; G, glucosamine; Inos, inositol; FA, fatty acid. (B) HeLa cells were first preincubated in a low-glucose medium with 6 μg/ml tunicamycin for 30 min at 37°C, then metabolically radiolabeled with [2-3H]mannose for 2.5 h at 37°C, and finally chased for 18 h in complete medium. At the end of the chase period, the cells were washed, incubated for 30 min on ice in PBS (without [−] or with [+] 10 mM sodium metaperiodate), and washed again in PBS supplemented with 150 mM glycerol. The cells were collected, and lipids were extracted and analyzed by TLC (chloroform-methanol-water, 10:10:3 [vol/vol/vol]). [3H]mannose-labeled H8 migrates with an Rf of ∼0.25 (the identity of H8 was confirmed by establishing its sensitivity to hydrolysis by GPI-specific phospholipase D [not shown]). Periodate treatment (lower panel) converts ∼50% of H8 to oxidized H8 (H8*). Several faster-migrating radiolabeled peaks were unaffected by periodiate, suggesting that these unidentified species are intracellular. (C) HeLa cells were first preincubated with myriocin (25 μg/ml) for 20 min, then pulse-labeled with [2-3H]mannose, and finally chased for 8 h in complete medium containing 62.5 μM myriocin (+ Myr). Control (untreated) (− Myr) and BFA-treated samples (cells were preincubated with BFA [2.5 μg/ml] and chased in the presence of the drug) were analyzed in parallel. Cells were taken at different time points and subjected to periodate oxidation to determine the fraction of radiolabeled H8 that was transported from the ER and located at the cell surface.