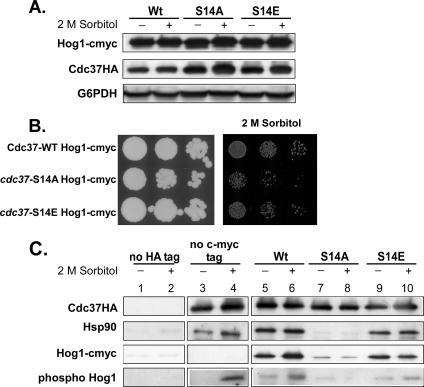
FIG. 5.
Effects of high osmotic stress on the interaction of Cdc37p with Hsp90 and Hog1p. (A) A C-terminal 13cmyc tag stabilizes Hog1p in the cdc37-S14A mutant. Strains PH401, PH402, and PH403, expressing HA-tagged wild-type Cdc37p and the S14A and S14E mutant versions, respectively, and 13cmyc epitope-tagged Hog1p were grown to mid-log phase in YPD medium and treated with 2 M sorbitol for 30 min. Cell extracts were prepared, and each extract was analyzed by Western blotting using anti-HA and an anti-cmyc antibody. An anti-G6PDH antibody was used as a loading control. (B) Hog1p-cmyc does not suppress the osmosensitive phenotype of the cdc37-S14A strain. Cells expressing wild-type Cdc37p or its S14A or S14E mutant version and containing 13cmyc epitope-tagged Hog1p (strains PH401, PH402, and PH403, respectively) were grown on YPD plates containing no additive or 2 M sorbitol. (C) Coimmunoprecipitation of Hsp90 and Hog1p with wild-type and mutant Cdc37p before and during osmotic stress. An aliquot of the same cell-free protein extracts analyzed in panel A was loaded onto an anti-HA agarose column (lanes 5 to 10). Cell extracts of strain PH404, containing 13cmyc-tagged Hog1 but no HA-tagged Cdc37 (lanes 1 and 2), and strain DH211, containing HA-tagged Cdc37Wt but no 13cmyc-tagged Hog1 (lanes 3 and 4), were used as controls. Bound protein was eluted by 2× SDS sample buffer. To avoid coelution of the heavy chain of the anti-HA antibody, which results in overlapping protein bands of endogenously phosphorylated Hog1 with the anti-HA heavy chain on a Western blot, bound protein of the control sample, containing endogenous Hog1, was eluted with 100 μg/ml HA peptide (see Materials and Methods). Fifteen percent of the total eluate was subjected to Western blot analysis using an anti-HA, anti-Hsp90, anti-phospho-Hog1p, or anti-cmyc antibody. The same blot was used for all analyses.