Abstract
We report here a case of congenital syphilis in a newborn after clindamycin treatment in pregnancy. Using PCR detection of tmpC (TP0319) and DNA sequencing of the genes TP0136 and TP0548, DNA sequences identical to Treponema pallidum subsp. pallidum strain SS14 were detected in the infant's skin lesions, serum, and cerebrospinal fluid.
CASE REPORT
A 2,200-g male infant was born by emergency cesarean section for placenta previa after a 35-week gestation to a 20-year-old secundigravida mother; the amniotic fluid was turbid.
The infant's mother was diagnosed with secondary syphilis in screening in the 15th week of pregnancy. She had a macular rash, and her serological findings were as follows: a rapid plasma reagin (RPR) test result of 1:64, microhemagglutination assay for antibodies to Treponema pallidum (MHA-TP) positive, fluorescent treponemal antibody-absorption (FTA-ABS) test positive, enzyme-linked immunosorbent assay (ELISA) immunoglobulin G (IgG) positive, and ELISA IgM positive. Her husband had no clinical signs of the disease at that time, but his serology was positive: RPR of 1:32, MHA-TP positive, FTA-ABS positive, ELISA IgG positive, and IgM negative. The mother was given clindamycin therapy due to a history of allergy to penicillin, which was not tested by a penicillin skin allergy test. There was a significant decrease in the RPR titer at the time of delivery. The serological findings were as follows: RPR of 1:16, MHA-TP positive, ELISA IgG positive, and ELISA IgM slightly positive.
The infant developed respiratory distress shortly after birth. He required mechanical ventilation and was therefore admitted to the neonatal intensive care unit (NICU) with a presumptive diagnosis of congenital syphilis. Upon admission to the NICU, examination revealed a liver palpable at 3 cm below the right costal margin in the midclavicular line, and a spleen palpable 2 cm below the left costal margin. Abdominal grayscale ultrasound showed no sonographic abnormalities of the hepatic parenchyma or the biliary structures. Long-bone radiographs demonstrated osteochondritis of both upper and lower extremities, a finding consistent with a diagnosis of congenital syphilis. Laboratory examination revealed a white blood cell count of 6.1 × 109 cells/liter (with 28% neutrophils), a hemoglobin level of 100 g/liter, and a platelet count of 11 × 109 cells/liter. The C-reactive protein level was 138.1 mg/liter.
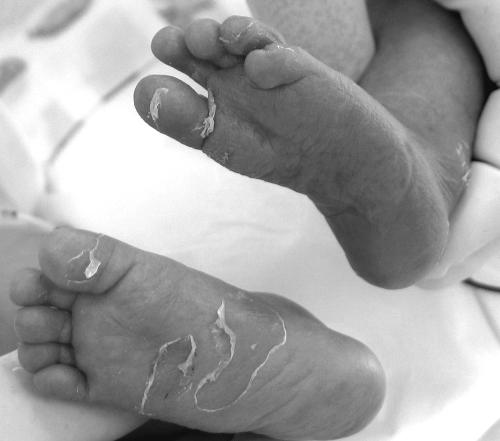
The infant's skin changes were the most significant with regard to the diagnosis of congenital syphilis. There were numerous vesicles with nonhemorrhagic content on his palms, fingers, soles, and crura. The vesicles on the infant's fingers and soles were stripped open, baring shallow erosions (Fig. 1). There were suppurative margins in the areas adjacent to the vesicles and shallow erosions and a discrete macular rash without desquamation on the patient's chest. No nasal discharge was presented.
FIG. 1.
The erosions on the infant's soles were swabbed, and DNA sequences identical to T. pallidum subsp. pallidum strain SS14 were identified in the samples using PCR detection of tmpC (TP0319) and DNA sequencing of the genes TP0136 and TP0548.
Therapy with aqueous crystalline penicillin G (100,000 U/kg/day) was initiated, and serological diagnostics and PCR of samples from skin lesions, cerebrospinal fluid (CSF), and serum were performed. Serological diagnostics included RPR, MHA-TP, FTA-ABS, ELISA IgG and IgM, and Western blot IgG and IgM.
Serological findings were as follows: RPR of 1:16, MHA-TP positive, FTA-ABS positive, ELISA IgM positive, ELISA IgG positive, Western blot IgM positive (antibodies to TpN15, TpN17, and TpN47), and IgG positive. CSF serological analysis was uninterpretable due to contamination with blood and the small sample of CSF taken.
A nested PCR protocol amplifying the tmpC gene (TP0319, which encodes a putative membrane lipoprotein) was used to detect T. pallidum subsp. pallidum in the clinical samples (3). Molecular analysis revealed T. pallidum subsp. pallidum in samples from the infant's skin lesions, serum, and CSF. Sequencing of the genes TP0136 and TP0548 in the PCR-positive samples revealed DNA sequences identical to T. pallidum subsp. pallidum strain SS14.
On postnatal day 8, mechanical ventilation was finished. On day 14, sepsis developed, and a diagnosis of hepatic insufficiency and ascites was made. A peripheral venipuncture blood culture yielded Enterobacter cloacae producing extended-spectrum β-lactamase. This culture showed sensitivity to chloramphenicol, ciprofloxacin, colistin, amikacin, and meropenem. The infant was started on ciprofloxacin therapy. On day 18, the patient's status rapidly worsened due to hepatic insufficiency, which ended in extreme hyperbilirubinemia (610 μmol/liter), and the infant died.
DNA from skin lesions, blood serum, and CSF was isolated by using a QIAamp DNA minikit (QIAGEN) according to the manufacturer's instructions. PCR detection of T. pallidum subsp. pallidum was performed as described previously (3). Briefly, the primers oTP0319F (5′-CTGCTCATCGGCTGCTCTA-3′) and oTP0319R (5′-ACCACAGACTTCGACCCATC-3′) were used to produce the first amplicon (773 bp) of nested PCR protocol detecting tmpC gene (TP0319). During the second step, 451-bp PCR product was amplified by using the following primers: iTP0319F (5′-GAAGGTGGTGACTTCGTCGT-3′) and iTP0319F (5′-CAAAACCCGCTTCAAAGAGA-3). Each PCR (25 μl) contained 0.125 μl of 10 mM concentrations of each deoxynucleoside triphosphates, 2.5 μl of 10× PCR buffer, 0.25 μl of each primer (100 pmol/μl), variable volumes of sterile distilled water (12 to 21 μl), and the examined DNA isolate (1 to 10 μl). To this reaction, 0.05 μl of Taq polymerase (5,000 U/ml; New England Biolabs) was added. PCRs were amplified as follows: 94°C for 1 min, followed by 30 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min, followed in turn by 72°C for 10 min. The second step of the PCR was performed identically with the following exceptions: 1 μl of the PCR containing the 773 bp amplicon was used as a template, and 40 amplification cycles were applied. Final amplicons were analyzed by using 2% agarose gel. Chromosomal DNA of T. pallidum subsp. pallidum comprising TP0136 and TP0548 genes was PCR amplified by using the primers TP0136F (5′-AGTGTCTTCCTCGTCCGTTC-3′) and TP0136R (5′-CACGTGGTGGTGTCAAACTT-3′), resulting in a 1,207-bp PCR product, and the primers TP0548F (5′-GCGGTCCCTATGATATCGTGT-3′) and TP0548R (5′-GAGCCACTTCAGCCCTACTG-3′), resulting in 1,066-bp amplicon. PCRs were set up as described above, and cycling conditions were as follows: 94°C for 1 min, followed by 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 2 min, and finally by 72°C for 10 min. The resulting PCR products were purified using QIAquick PCR purification kit (QIAGEN) and subjected to dideoxy terminator sequencing using amplification primers and additional internal primers. Sequence analysis was performed in DNASTAR software (Lasergene).
The number of treponemal DNA copies (inferred from the maximal dilution of PCR-positive samples) was identical in serum and CSF and came to approximately 106 DNA molecules per 1 ml of undiluted serum and CSF samples.
Treponemal DNA in congenital syphilis can be detected in a number of clinical samples including serum, whole blood, amniotic fluid, paraffin-embedded placental tissue, bullous targetoid skin lesions, and CSF (4, 7, 9, 11). Detection of treponemal DNA in whole blood and serum in patients with congenital syphilis appears to be more reliable than in adult patients (7, 9). This is probably due to the relatively higher concentration of treponemes in the blood of these patients. In our case, the number of treponemal DNA copies emphasizes the fact that in early congenital syphilis the concentration of treponemes in serum and CSF is similar and notably higher than in adults (3). These findings are consistent with the study by Michelow et al. in which central nervous system involvement in infants with congenital syphilis was best predicted by IgM immunoblotting of serum or a PCR assay of serum or blood (7).
Skin lesions either in the form of a typical vesiculobullous eruption (especially over the palms of the hands and the soles of the feet) or a maculopapular skin rash over the body are common presentations of early congenital syphilis and have been described in several studies (5, 6, 10, 11). In a study of premature infants with congenital syphilis, 62% had unusual desquamation over palms and soles (6). Bone changes, hepatosplenomegaly, respiratory distress, CSF abnormalities, and jaundice are the other major manifestations of the disease in premature infants (6). Moreover, syphilis is highly probable in all infants with a serum quantitative nontreponemal serologic titer that is fourfold greater than the mother's titer (1). In the case presented here, the infant's serum RPR titer (1:16) was identical to the mother's RPR titer in serum samples that were taken immediately after delivery. There was a fourfold decrease of the initial mother's RPR titer after treatment. Despite the decrease, the infant was infected because of inadequate maternal treatment.
Parenteral penicillin G is the only therapy with documented efficacy for syphilis during pregnancy. In experiment on rabbits, single intramuscular doses of clindamycin (15 or 40 mg/kg) did not decrease treponemal counts significantly, but single injections of penicillin (10,000 U/kg) reduced treponemal counts by more than 250-fold. Multiple intramuscular injections of clindamycin reduced counts by five- to sevenfold, whereas multiple doses of penicillin decreased treponeme counts by greater than 300-fold. Despite partially crossing the placenta, clindamycin is far less effective than penicillin in treating syphilitic lesions (2, 8). Pregnant women with syphilis who report a penicillin allergy should be desensitized and treated with penicillin. Skin testing may be useful in pregnant women to establish whether a penicillin allergy exists (1).
In conclusion, this report documents the importance of treating syphilis during pregnancy with penicillin and endorses the use of molecular techniques to identify T. p. pallidum in clinical samples to diagnose congenital early syphilis.
Acknowledgments
This work was supported by a grant from the Ministry of Health of the Czech Republic (NR8967-4/2006), by a grant of the Grant Agency of the Czech Republic (310/04/0021), and by institutional support of the Ministry of Education of the Czech Republic (VZ MSM0021622415).
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Atkins, D., K. A. Workowski, et al. 2006. Sexually transmitted diseases treatment guidelines, 2006. Morb. Mortal. Wkly. Rep. 55:1-94. [Google Scholar]
- 2.Brause, B. D., J. S. Borges, and R. B. Roberts. 1976. Relative efficacy of clindamycin, erythromycin, and penicillin in treatment of Treponema pallidum in skin syphilomas of rabbits. J. Infect. Dis. 134:93-96. [DOI] [PubMed] [Google Scholar]
- 3.Flasarova, M., D. Smajs, P. Matejkova, V. Woznicova, M. Heroldova-Dvorakova, and M. Votava. 2006. Molecular detection and subtyping of Treponema pallidum subsp. pallidum in clinical specimens. Epidemiol. Mikrobiol. Immunol. 55:105-111. [PubMed] [Google Scholar]
- 4.Grimprel, E., P. J. Sanchez, G. D. Wendel, J. M. Burstain, G. H. McCracken, J. D. Radolf, and M. V. Norgard. 1991. Use of polymerase chain reaction and rabbit infectivity testing to detect Treponema pallidum in amniotic fluid, fetal and neonatal sera, and cerebrospinal fluid. J. Clin. Microbiol. 29:1711-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh, M. T., and C. T. Lim. 1991. Early congenital syphilis: experience with 13 consecutive cases seen at the University Hospital, Kuala Lumpur. Singapore Med. J. 32:230-232. [PubMed] [Google Scholar]
- 6.Liu, C. C., W. C. So, C. H. Lin, and T. F. Yeh. 1993. Congenital syphilis: clinical manifestations in premature infants. Scand. J. Infect. Dis. 25:741-745. [DOI] [PubMed] [Google Scholar]
- 7.Michelow, I. C., G. D. Wendel, M. V. Norgard, F. Zeray, N. K. Leos, R. Alsaadi, and P. J. Sanchez. 2002. Central nervous system infection in congenital syphilis. N. Engl. J. Med. 346:1792-1798. [DOI] [PubMed] [Google Scholar]
- 8.Meljanac, N., E. Dippel, and C. C. Zouboulis. 1999. Superimposed primary chancre in a patient with Adamantiades-Behcet's disease. Sex. Transm. Infect. 75:124-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez, P. J., G. D. Wendel, Jr., E. Grimprel, M. Goldberg, M. Hall, O. Arencibia-Mireles, J. D. Radolf, and M. V. Norgard. 1993. Evaluation of molecular methodologies and rabbit infectivity testing for the diagnosis of congenital syphilis and neonatal central nervous system invasion by Treponema pallidum. J. Infect. Dis. 167:148-157. [DOI] [PubMed] [Google Scholar]
- 10.Wenhai, L., Z. Jianzhong, and Y. Cao. 2004. Detection of Treponema pallidum in skin lesions of secondary syphilis and characterization of the inflammatory infiltrate. Dermatology 208:94-97. [DOI] [PubMed] [Google Scholar]
- 11.Wu, C. C., C. N. Tsai, W. R. Wong, H. S. Hong, and Y. H. Chuang. 2006. Early congenital syphilis and erythema multiforme-like bullous targetoid lesions in a 1-day-old newborn: detection of Treponema pallidum genomic DNA from the targetoid plaque using nested polymerase chain reaction. J. Am. Acad. Dermatol. 55:S11-15. [DOI] [PubMed] [Google Scholar]