Figure 6. Truncated HDAC3 (aa 1–391) Is Recruited to the c-jun Promoter Region and Is Responsible for Sorbitol-Induced Transcriptional Repression of c-jun.
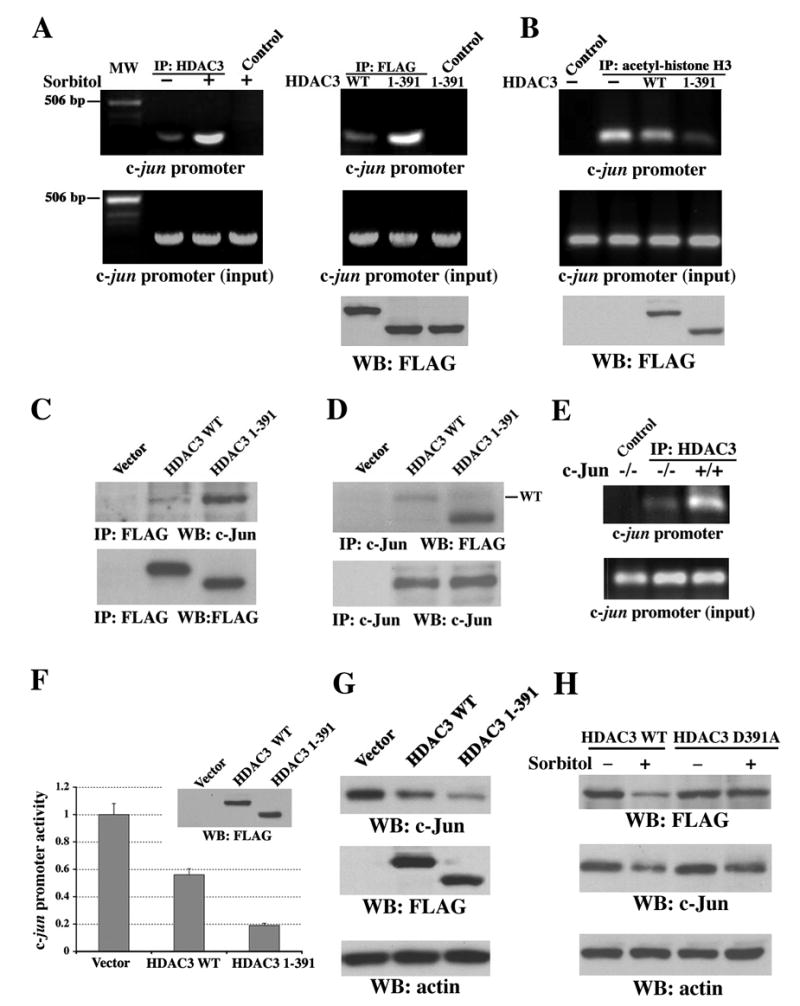
(A–D and F–H) Immunoblotting analyses with the indicated antibodies.
(A) Chromatin was prepared from NIH3T3 cells (left) treated with or without sorbitol for 3 h or from 293T cells (right) transiently expressing WT FLAG-HDAC3 or FLAG-HDAC3 (aa 1–391). ChIP analysis was carried out with primers for mouse (left) or human (right) c-jun promoter. A rabbit anti-HDAC3 antibody (H-99), mouse anti-FLAG antibody, or normal rabbit or mouse IgG as a control was used for immunoprecipitation. MW, molecular weight.
(B) 293T cells transiently expressing with or without WT FLAG-HDAC3 or FLAG-HDAC3 (aa 1–391) were used for ChIP analysis with an acetyl-histone H3 antibody for immunoprecipitation.
(C–D) 293T cells transiently expressing with or without WT FLAG-HDAC3 or FLAG-HDAC3 (aa 1–391) were used for immunoprecipitation with anti-FLAG (C) or anti-c-Jun (D) antibodies.
(E) Chromatin prepared from c-Jun−/− and c-Jun+/+ cells, which were treated with sorbitol for 3 h, were used for ChIP analysis with a rabbit anti-HDAC3 antibody (H-99) or normal rabbit IgG as a control for immunoprecipitation.
(F) pFLAG (vector), pFLAG-HDAC3 WT, or pFLAG-HDAC3 (aa 1–391) was transiently transfected into 293T cells expressing pc-Jun-Luc and subjected to luciferase-reporter and immunoblotting analyses with an anti-FLAG antibody. The relative levels of luciferase activity were normalized to the levels of pFLAG-transfected cells and to the levels of luciferase activity of the Renilla control plasmid. Data represent the means ± SD of three independent experiments.
(G) 293T cells were transiently transfected with pFLAG, pFLAG-HDAC3 WT, or pFLAG-HDAC3 (aa 1–391).
(H) NIH 3T3 cells transiently transfected with pFLAG-HDAC3 WT or pFLAG-HDAC3 D391A and were treated with or without sorbitol for 6 h.
