Figure 5.
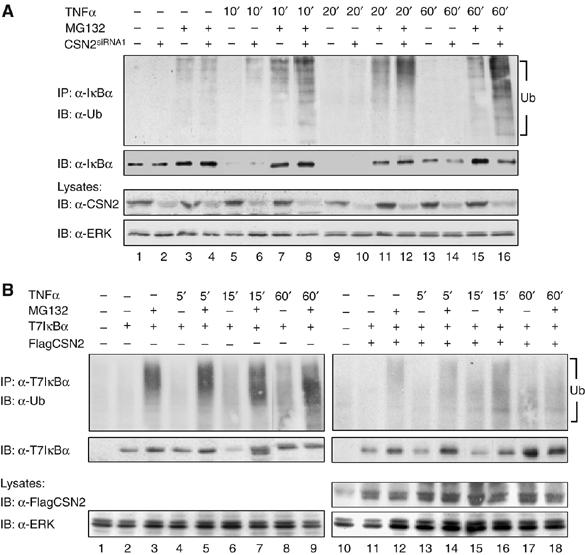
CSN promotes deubiquitinylation of IκBα. (A) Cells were either transiently transfected with CSN2siRNA1 (lanes 2, 4, 6, 8, 10, 12, 14 and 16) or mock transfectd (lanes 1, 3, 5, 7, 9, 11, 13 and 15). Then, cells were stimulated for different periods of time with TNFα or left untreated as indicated. At 1 h before stimulation, MG132 (lanes 3, 4, 7, 8, 11, 12, 15 and 16) or vehicle (DMSO, lanes 1, 2, 5, 6, 9, 10, 13 and 14), was added to the culture medium. After harvest of the cells by RIPA lysis, endogenous IκBα was immunoprecipitated, using an anti-IκBα antibody. Ubiquitinylated IκBα was immunodetected with an anti-ubiquitin antibody. The blot was stripped and developed with an anti-IκBα antibody to show TNFα-induced degradation and re-accumulation of IκBα. In addition, CSN2 was immunodetected in the cell lysates, using an anti-CSN2 antibody, to verify efficient knockdown of the CSN2 protein. Immunodetection of ERK in the cell lysates was performed as protein load control. (B) T7IκBα was expressed either alone (lanes 2–9) or in combination with FlagCSN2 (lanes 11–18) by transient transfection of equal amounts of cDNA in HeLa cells. Before cell lysis, the cells were stimulated with TNFα (lanes 4–9 and 13–18) for the indicated periods of time or left untreated (lanes 1–3 and 10–12). Additionally, the cells were treated for 1 h with either MG132 (lanes 3, 5, 7 9, 12, 14, 16 and 18) or vehicle (DMSO, lanes 1, 2, 4, 6, 8, 10, 11, 13, 15 and 17) before stimulation. Ectopically expressed T7IκBα was immunoprecipitated from cell lysates using an anti-T7 antibody and the protein complexes were subjected to SDS–PAGE and Western blotting. T7IκBα ubiquitinylation in TNFα-stimulated cells was determined by immunostaining with an anti-ubiquitin antibody (upper panels). The stripped immunoblots were developed with an anti-IκBα antibody to detect the TNFα-induced proteolytic turnover of T7IκBα, which was inhibited by MG132 (second panels). Ectopic expression of FlagCSN2, which mediates enhanced expression of CSN, was recognised by immunodetection with an anti-Flag antibody in the total cell lysate. Immunodetection of ERK was performed as protein load control.
