Figure 6.
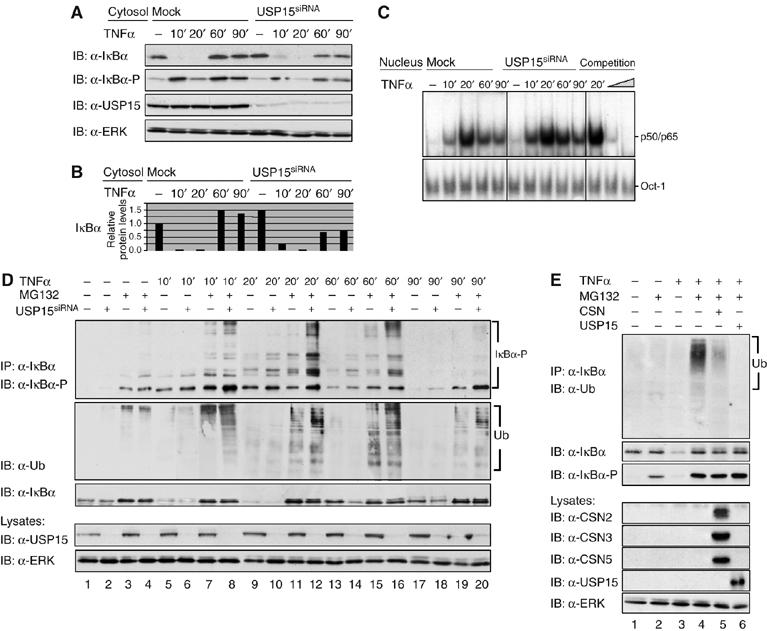
CSN-associated USP15 deubiquitinylates IκBα. (A) Cells were either transiently transfected with USP15siRNA or mock transfected. After 48 h, cells were stimulated for different periods of time, as indicated, with TNFα. Cytosolic fractions of USP15-knockdown cells were analysed by SDS–PAGE and Western blotting. To confirm TNFα-induced phosphorylation and turnover of IκBα, immunodetection of IκBα and its Ser32/Ser36-phosphorylated form was performed using specific antibodies. USP15 was immunodetected to confirm knockdown efficiency. Immunodetection of ERK in the cell lysates was performed as protein load control. (B) Quantification of the results shown for IκBα in (A) was performed as described in Figure 2B. (C) Nuclear fractions were analysed by EMSA as described in Figure 3F. (D) Cells were either transiently transfected with USP15siRNA (lanes 2, 4, 6, 8, 10, 12, 14, 16, 18 and 20) or mock-transfected (lanes 1, 3, 5, 7, 9, 11, 13, 15, 17 and 19). Then, cells were stimulated for different periods of time with TNFα (lanes 5–20) or left untreated (lanes 1–4). At 1 h before stimulation, MG132 (lanes 3, 4, 7, 8, 11, 12, 15, 16, 19 and 20) or vehicle (DMSO, lanes 1, 2, 5, 6, 9, 10, 13, 14, 17 and 18) was added to the culture medium. After harvest of the cells, endogenous IκBα was immunoprecipitated from cytosolic fractions of HeLa cells using an anti-IκBα antibody. Immunoprecipitates were analysed by SDS–PAGE and Western blotting. The IκBα protein and its multiple high-molecular-weight forms of phosphorylated IκBα were immunodetected using IκBα- and phospho-IκBα-specific antibodies. Ubiquitinylated IκBα was immunodetected with an anti-ubiquitin antibody. USP15 was immunodetected in the cell lysates, using an anti-USP15 antibody, to verify efficient knockdown of the USP15 protein. Immunodetection of ERK in the cell lysates was performed as protein load control. (E) Cells were preincubated for 1 h (lanes 2 and 4–6) with MG132 or treated with vehicle (DMSO, lanes 1 and 3) and subsequently stimulated (lanes 3–6) or not (lanes 1 and 2) with TNFα for 15 min. IκBα immunoprecipitates were incubated for 4 h at 37°C in the presence of an ATP regenerating system. In addition, purified CSN (lane 5) or recombinant USP15 (lane 6) was added to catalyse deubiquitinylation of IκBα. Aliquots of the reactions were analysed by SDS–PAGE and Western blotting. Immunodetection of ubiquitin, IκBα and its Ser32/Ser36-phosphorylated form, CSN subunits and USP15 (see indicated panels) was performed using specific antibodies. Immunodetection of ERK in the cell lysates was used as a control for equal amounts of protein applied to each immunoprecipitation.
