Figure 7.
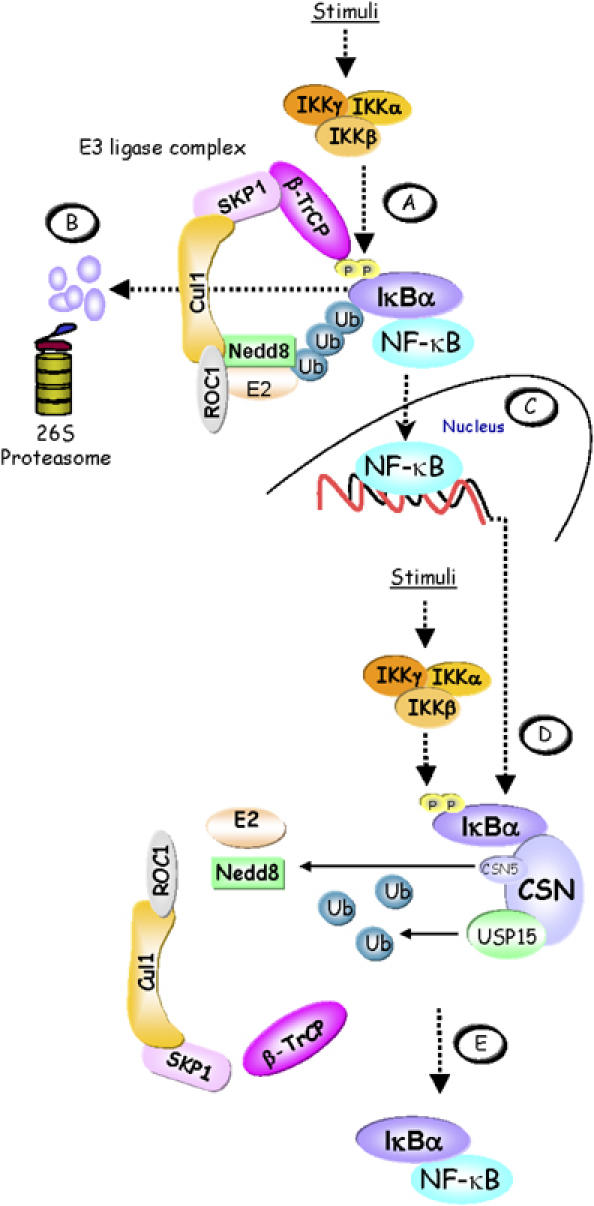
Model of CSN-mediated IκBα control. (A) In stimulated cells, the activated IKK complex phosphorylates Ser32 and Ser36 in the N-terminus of IκBα. When phosphorylated, these serine residues are part of a ‘degron' that is specifically recognised by β-TrCP, the CRL substrate adapter for IκBα. CRL activity is promoted by neddylation of Cul1. The latter facilitates the recruitment of a ubiquitin-loaded ubiquitin-conjugating enzyme (E2) to the ROC1 subunit of the CRL, which then cooperates with the CRL to ubiquitinylate IκBα. (B) Ubiquitinylated IκBα is degraded via the 26S proteasome releasing the previously bound and inactivated NF-κB. (C) Released NF-κB translocates to the nucleus to activate target genes, including the IκBα gene. As part of a negative feedback loop, re-accumulating IκBα can dissociate DNA-bound NF-κB and re-transport it to the cytosol. (D) During persistent stimulation, re-accumulated IκBα becomes phosphorylated in the cytosol by the IKK complex, thus initiating the cycle again. However, the CSN can enhance the stability of IκBα and thereby contribute to shut down NF-κB activity. This might be brought about in part by cycles of CSN5-mediated deneddylation of Cul1, which controls CRL activity. Most importantly, however, IκBα is deubiquitinylated by the CSN-associated DUB USP15. (E) Re-accumulated IκBα firmly associates with NF-κB, keeping it inactive in the cytosol.
