Figure 1.
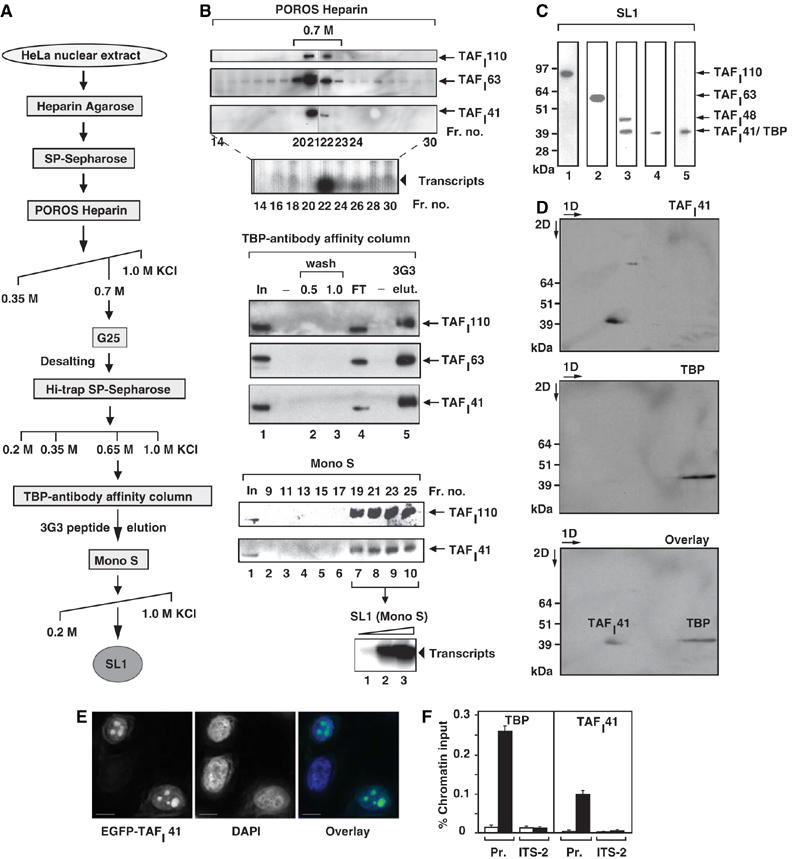
Human nucleolar protein TAFI41 co-purifies with TBP-antibody affinity-purified SL1 and is found at the rDNA promoter in human cells. (A) Human SL1 purification scheme. For analytical purification of SL1, HeLa cell nuclear extract was applied to POROS Heparin Agarose and SP-Sepharose columns, as previously described (Comai et al, 1992; Friedrich et al, 2005), then salt gradient eluted from a POROS Heparin column. The SL1 fractions were pooled, desalted on G25 Sepharose, step-eluted from SP-Sepharose and applied to a TBP-antibody affinity column. SL1 complexes were eluted with an excess of TBP (3G3) peptide and this eluate was applied to a Mono S column, which was developed by a linear salt gradient (KCl at concentrations (M) as indicated). (B) TAFI41 co-purifies with SL1 protein and activity from HeLa cell nuclear extracts. Fractions obtained by salt gradient elution from the POROS Heparin column (B) were analysed for SL1 protein by immunoblotting, with antibodies specific for SL1 subunits TAFI110 and TAFI63, and an antibody generated against three peptides of TAFI41 (see Materials and methods). The bulk of TAFI41 eluted with the other SL1 subunits at 0.7 M KCl (fractions 21 and 22). Fraction 22 supported specific SL1-dependent reconstituted Pol I transcription. The 0.7 M KCl fraction was desalted to 0.2 M KCl, passed over SP-Sepharose and a salt-step elution was performed. The bulk of SL1 including TAFI41 eluted at 0.65 M KCl and supported specific SL1-dependent transcription. This SL1 fraction was loaded onto a TBP-antibody affinity column (input, lane 1), some of which flowed through (FT, lane 4). The column was washed at 0.5 and 1.0 M KCl (lanes 2 and 3). Bound proteins from the washed column were eluted with excess TBP-epitope peptide 3G3 (1 mg/ml) (lane 5) and fractions were immunoblotted for SL1 with antibodies specific for TAFI110 and TAFI63, and the TAFI41-peptides antibody. SL1 was concentrated and purified away from the 3G3-peptide on a Mono S column, developed with a linear salt gradient. The Mono-S purified SL1 fractions 19–25 (fractions 19, 21, 23 and 25 are shown in lanes 7–10) were pooled and tested for the ability to support SL1-dependent transcription with UBF and Pol I (lanes 1–3, arrowhead). (C) TBP and TAFI41 display similar apparent molecular weights. SL1 (the 0.7 M fraction from the POROS-Heparin column, see A, B) was immunoblotted with antibodies specific for TAFI110, TAFI63, TAFI48 and TBP (top panel, lanes 1, 2, 3 and 5, respectively) and the TAFI41-peptides antibody (lane 4). A band of similar mobility to those of TBP and TAFI41, detected by TAFI48 antibodies, is either a degradation product of TAFI48 or could represent a protein encoded by a splice variant 2 of TAFI48 (top panel, lane 3). (D) TBP and TAFI41 are separable by two-dimensional gel electrophoresis. SL1 (the 0.7 M fraction from the POROS-Heparin column, see A and B) was subject to first dimension isoelectric focusing (pH 3–10), then to second dimension separation (4–12% Bis–Tris gradient gel, Invitrogen). SL1 proteins were immunoblotted, probed first with TAFI41-peptides antibody (upper panel) and then with antibodies specific for TBP (middle panel). The lower panel shows an overlay of the signals from these immunoblots. (E) Overexpressed TAFI41 accumulates in nucleoli. HeLa cells were transfected with expression vectors pEGFP-TAFI41. After 24 h, the cells were fixed and viewed by confocal microscopy. DNA was stained with DAPI. Overlay is a composite of the DAPI (blue) and EGFP-TAFI41 (green) signals. Scale bar, 10 nm. (F) ChIP analysis indicates that TAFI41 is present at the promoter of the rRNA genes. ChIP analysis (from HeLa cells) used TAFI41-peptides antibody (and as a control rabbit preimmune serum) or a TBP mouse monoclonal antibody (and as a control mouse IgG), followed by quantitative real-time PCR with primers specific for the promoter region and the internal transcribed spacer (ITS-2) of the human rDNA (see Materials and methods). The relative levels of rDNA associated with TAFI41 and TBP at these sites are expressed as percentage of input chromatin and are from two independent experiments.
