Figure 1.
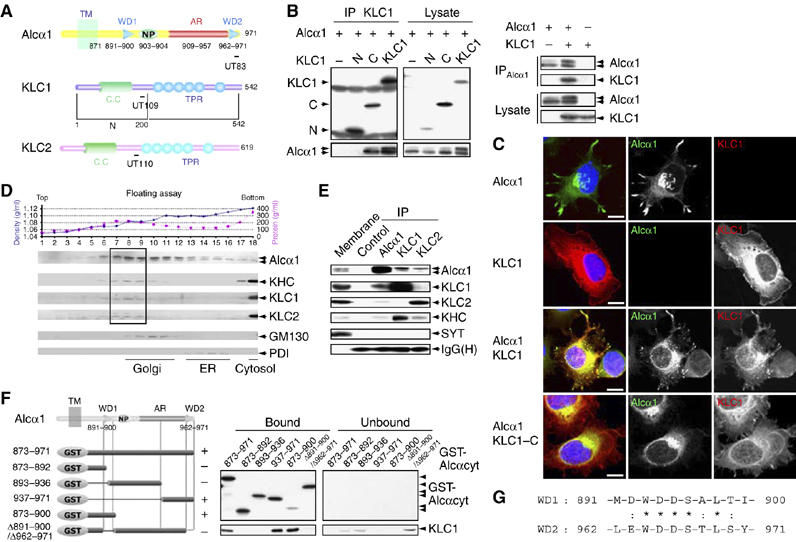
Interaction of Alcα with KLC and determination of the KLC-binding site on Alcα. (A) Structure of the cytoplasmic domains of Alcα, KLC1, KLC1-N (N), KLC1-C (C), and KLC2. Numbers represent amino-acid sequences. TM, transmembrane domain; WD, tryptophan- and aspartic acid-containing sequence (see ‘G'); NP, Asn-Pro motif; AR, acidic region; CC, coiled-coil domain; TPR, tetratrico-peptide repeat. Epitopes for the UT83, UT109, and UT110 antibodies are indicated with bars. (B) (Left panel) Co-immunoprecipitation of Alcα1 with amino-terminal FLAG-tagged KLC1 and deletion constructs. HEK293 cells were transiently cotransfected with pcDNA3-hAlcα1 and pcDNA3.1-FLAG-mKLC1 (KLC1), pcDNA3.1-FLAG-mKLC1-N (N), pcDNA3.1-FLAG-mKLC1-C (C), or pcDNA3.1-FLAG (−). Cell lysates were immunoprecipitated with an anti-FLAG (M2) antibody. The immunoprecipitates (IP) and lysate were analyzed by Western blotting with M2 and UT83. (Right panel) Co-immunoprecipitation of KLC1 with Alcα1. HEK293 cells were transiently transfected with pcDNA3-hAlcα1 and pcDNA3.1-mKLC1. Transfection with plasmid (+) or vector alone (−) is indicated. Cell lysates were immunoprecipitated with an anti-Alcα (UT83) antibody. The immunoprecipitates (IP) and lysate were analyzed by Western blotting with UT83 and UT109. (C) Localization of Alcα1 and KLC1. CAD cells were transiently cotransfected with the indicated combinations of pcDNA3-hAlcα1, pcDNA3.1-FLAG-mKLC1, and pcDNA3.1-FLAG-mKLC1-C for 48 h and differentiated for 24 h by depleting serum. Alcα1 and FLAG-tagged KLC1 were immunostained with UT83 (green) and M2 (red) antibodies, respectively. Nuclei were stained using 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI, blue). Merged signals are shown at the left. Scale bar, 5 μm. (D) Membrane fractionation of brain tissues. The post-nuclear supernatant of adult mouse brains was fractionated by 0–28% iodixanol density gradient centrifugation (Araki et al, 2003). (Upper panel) The density (blue circles) and protein concentration (pink squares) are indicated. Fraction numbers are indicated along the abscissa. (Lower panel) The fractions were analyzed by Western blotting with antibodies to Alcα (UT83), KHC (H2), KLC1 (UT109), KLC2 (UT110), and the Golgi-resident GM130 (clone no. 35) and the ER-resident PDI (1D3). The dotted square indicates the fractions containing the highest levels of Alcα, KHC, KLC1, and KLC2. (E) Co-immunoprecipitation of Alcα with KLC. The mouse brain membrane fraction (500 μg protein) was solubilized, and Alcα1, KLC1, and KLC2 were immunoprecipitated with the UT83, UT109, and UT110 antibodies, respectively, or a control non-immune antibody. The membrane fraction (Membrane, 10 μg protein) and immunoprecipitates (IP) were analyzed by Western blotting with antibodies specific for Alcα (UT83), KLC1 (UT109), KLC2 (UT110), KHC (H2), and SYT (clone no. 41). IgG(H) indicates the IgG heavy chain (rabbit). (F) Determination of the KLC-binding site on Alcα. (Left) Various GST-fusion proteins of the Alcα cytoplasmic domain (shown schematically, numbers are amino-acid residues) were incubated with HEK293 cell lysates expressing FLAG-KLC1. FLAG-KLC1 bound to GST-Alcαcyt was recovered using glutathione beads. (Right) The bound and unbound FLAG-KLC1 were analyzed by Western blotting with the M2 antibody. GST-Alcα1 protein constructs were detected by Western blotting with an anti-GST antibody. In the left panel, ‘+' indicates positive binding and ‘−' indicates negative binding. (G) Amino-acid sequences of WD1 and WD2 of Alcα1. Numbers indicate amino-acid residues. Identical (*) and similar (:) amino-acid residues are indicated.
