Figure 6.
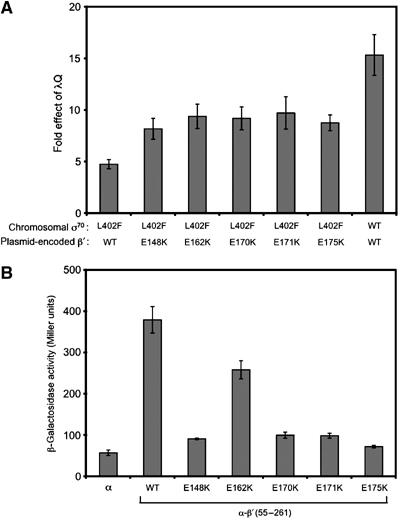
Substitutions in β′ suppress the defect in λQ-mediated antitermination caused by the σ70 L402F substitution and weaken the interaction between the β′ SNCRID and the σ70 NCR. (A) Cells encoding either σ70 L402F or WT σ70 at the chromosomal rpoD locus and harboring the PR′-lacZ fusion on an F′ episome were cotransformed with compatible plasmids, one directing the synthesis of the indicated β′ protein (in addition to chromosomally encoded WT β′) and the other directing the synthesis of either λQ or no λQ. Cells were grown in the presence of 100 μM IPTG and assayed for β-galactosidase activity. ‘Fold effect of λQ' values were calculated as described for Figure 1. Shown are the averages of three independent sets of measurements (and standard deviations). (B) Effects of substitutions in the β′SNCRID on the interaction between the β′ moiety of the α–β′55–261 fusion protein and the σ moiety of the λCI-σ70 94–448 fusion protein. Strain FW102 F′OL2–62 cells containing compatible plasmids directing the synthesis of the λCI-σ70 fusion protein and either α or the indicated α–β′ fusion protein were grown in the presence of 20 μM IPTG and assayed for β-galactosidase activity. Assays were performed three times in duplicate on separate occasions; shown are the average values from all trials with standard deviations.
