Abstract
Heart valve replacements fabricated from glutaraldehyde (Glut)-crosslinked heterograft materials, porcine aortic valves or bovine pericardium, have been widely used in cardiac surgery to treat heart valve disease. However, these bioprosthetic heart valves often fail in long-term clinical implants due to pathologic calcification of the bioprosthetic leaflets, and for stentless porcine aortic valve bioprostheses, bioprosthetic aortic wall calcification also typically occurs. Previous use of the epoxide-based crosslinker, Triglycidyl amine (TGA), on cardiac bioprosthetic valve materials demonstrated superior biocompatibility, mechanics, and calcification resistance for porcine aortic valve cusps (but not porcine aortic wall) and bovine pericardium, versus Glut-prepared controls. However, TGA preparation did not completely prevent long-term calcification of cusps or pericardium. Herein we report further mechanistic investigations of an added therapeutic component to this system, 2-Mercaptoethylidene-1,1-bisphosphonic acid (MABP), a custom synthesized thiol bisphosphonate, which has previously been shown in a preliminary report to prevent bioprosthetic heterograft biomaterial calcification when used in combination with initial TGA crosslinking for 7 days. In the present studies we have further investigated the effectiveness of MABP in experiments that examined: 1) The use of MABP after optimal TGA crosslinking, in order to avoid any competitive interference of MABP-reactions with TGA during crosslinking; 2) Furthermore, recognizing the importance of alkaline phosphatase in the formation of dystrophic calcific nodules, we have investigated the hypothesis that the mechanism by which MABP primarily functions is through the reduction of alkaline phosphatase activity. Results from cell-free model systems, cell culture studies, and rat subcutaneous implants, show that materials functionalized with MABP after TGA crosslinking have reduced alkaline phosphatase activity, and in vivo have no significant calcification in long term implant studies. It is concluded that bioprosthetic heart valves prepared in this fashion are compelling alternatives for Glut-prepared bioprostheses.
1. Introduction
Glutaraldehyde (Glut) is a well-known protein crosslinking agent. In the preparation of clinically used heterograft bioprosthetic heart valves (porcine aortic valves or bovine pericardium), Glut is used as a fixative due to its principal roles as stabilizer [1] and sterilant [2], with some additional hypothetical benefits in the reduction of immunogenicity [3,4]. Despite these benefits, Glut crosslinks are inherently unstable [5,6] and as a result, a cytotoxic microenvironment ensues [7,8]. Moreover, Glut-prepared bioprosthetic heart valves have a finite clinical lifetime, ultimately failing, most commonly due to pathological calcification [9].
Our group has previously reported an alternative protein stabilization strategy based on the use of the polyepoxide crosslinker, Triglycidyl amine (TGA) [10]. Optimization experiments with TGA determined that a 7-day crosslinking protocol was necessary for maximal tissue stabilization and collagenase resistance [10]. Rat subcutaneous implant studies with TGA-prepared materials demonstrated significant calcification resistance over Glut controls for porcine cusp and bovine pericardial implants, but not porcine aortic wall [11].
Due to the inability of TGA alone to completely mitigate calcification, a second strategy was investigated by our group wherein an anticalcific bisphosphonate (2-Mercaptoethylidene-1,1-bisphosphonic acid, MABP) was synthesized, and hypothesized to act synergistically with covalent attachment during TGA crosslinking in functionalizing heterograft materials [11]. Bisphosphonates are calcium-binding compounds that associate strongly with calcium phosphate minerals (including hydroxyapatite) and prevent both crystal formation and dissolution [12] as well as interfere with the osteogenesis related enzyme, alkaline phosphatase [13].
Prior work on bisphosphonates had demonstrated their efficacy in similar milieus [14,15], and in a preliminary report we noted that combining TGA and MABP during each of the 7 days of TGA-crosslinking demonstrated amelioration of calcification in bioprosthetic heart valve biomaterials [11]. However, we hypothesize that a terminal incubation with MABP after the conclusion of TGA crosslinking will suitably functionalize materials with MABP, pose no competitive risk to crosslinking, achieve effective bisphosphonate incorporation, and yield optimal calcification prevention. Furthermore, we hypothesize that the principal mechanism of action for MABP in this setting involves alkaline phosphatase inhibition. Lastly, we predict that crosslinking with TGA, and terminally pretreating with MABP, will maintain characteristic tissue morphology in implanted materials for extended periods of time.
Therefore, the present studies had the following objectives:
To investigate covalent attachment of MABP to TGA crosslinked heterograft biomaterials as a terminal procedure following TGA crosslinking, rather than concomitantly as previously reported.
To examine MABP incorporation into TGA crosslinked collagen, and both porcine aortic valve cusps and aortic wall, for inhibition of alkaline phosphatase (ALP) activity in cell free systems, cell culture, and in vivo.
To investigate the efficacy of MABP incorporated terminally into TGA-pretreated porcine aortic cusps and aortic wall for inhibiting pathologic calcification in rat subdermal implant studies.
2. Methods
2.1 Materials TGA, MABP
Triglycidyl amine (TGA) was primarily synthesized as previously described [10], in brief reacting aqueous ammonia with excess epichlorohydrin. Following several additional reaction steps, a novel cold crystallization technique resulted in a coarse white water-soluble powder. Synthesis of 2-(2-mercaptoethylamino)ethylidene-1,1-bisphosphonic acid (MABP) involves the initial reaction of vinylidene bisphosphonic acid in excess cystamine resulting in a coarse white crystalline powder [11]. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
2.2 Collagen substrate and plate preparation
Collagen culture substrates were prepared in tissue culture plates by gelation of neutralized native type I collagen (Inamed, Inc., Fremont, CA) according to manufacturer’s directions. The collagen volume of each cell culture plate’s collagen coating was normalized to 0.158 ml/cm2 of growth surface area, and stored under normal saline at 37°C until use. TGA preparation of collagen gels was performed as previously described using 100mM TGA in pH 7.4 borate mannitol buffer, followed by neutralization of residual epoxy groups with 100mM thiosulfate [10]. For sequential TGA/MABP collagen, neutralization was preceded by an overnight incubation in 20mM MABP in borate mannitol buffer (pH=7.4). The plates were then again washed three times with sterile normal saline, and residual epoxy groups neutralized by subsequent overnight incubation at 37°C with 0.1 mol/L sodium thiosulfate in borate mannitol buffer (pH=7.4). Lastly, after washing three times with sterile normal saline, plates were stored under normal saline at 37°C until use.
2.3 Acellular measurement of alkaline phosphatase activity
0.1U, 1U, and 2.5U of alkaline phosphatase (P6774; Sigma-Aldrich) was added to wells in a standard 6 well plate, which had been prepared with TGA/MABP pretreated collagen as a sequential MABP linkage as described above, TGA/MABP simultaneous linkage, or TGA alone pretreated collagen. Following a 4 hour incubation, ALP activity was measured at 405nm spectrophotometrically using the p-Nitrophenyl Phosphate (pNPP, Sigma-Aldrich) substrate system per the manufacturer’s specifications.
2.4. Cells and cell culture
Normal human aortic valves from explanted hearts were obtained at heart transplant surgery under an exemption approved by the IRB of the Hospital of the University of Pennsylvania. Sheep Aortic Valve Interstitial Cells (SAVICs) and Human Aortic Valve Interstitial Cells (HAVICs) were isolated as previously described [16] and used between passages 2 and 10. Cells were routinely cultured in M199 (Life Technologies, Inc., Grand Island, NY) supplemented with 10% fetal bovine serum (Gemini Bio-Products, Woodland CA) and penicillin/streptomycin (Life Technologies, Inc.). Cells were plated at a density normalized to 5.3 x 104 cells/cm2 on 6 well plates coated with non-crosslinked collagen, TGA-prepared collagen, and TGA/MABP-prepared collagen, prepared as mentioned above. Following three days of incubation at 37°C, cells were histochemically stained for ALP activity as described below. Immediately after stain development, digital images were taken for each well. The images were processed as previously described [17], in Adobe Photoshop to eliminate background and converted into binary (black and white) images by using the program IMAGE (Scion, Frederick, MD). The area of “black” pixels representing ALP staining was divided by the total analyzed area and expressed as a value that was normalized to a full scale of 5 arbitrary units.
2.5 Alkaline phosphatase histochemistry
5-Bromo-4-Chloro-3-Indolyl-Phosphate (BCIP) and 4-Nitro Blue Tetrazolium Chloride (NBT) solutions were obtained from Roche Diagnostics (Indianapolis, IN). To stain cell culture plates with BCIP/NBT, plates were first washed three times with 1x PBS (w/Ca & Mg). Cells were then fixed w 4% PFA for 10 minutes and washed three more times with 1x PBS (w/Ca & Mg). A solution consisting of 5ml of 1M Tris-HCl buffer aliquots. Plates were then incubated overnight at 37°C and rinsed with 1x PBS (w/Ca & Mg) prior to visualization with light microscopy.
2.6 Sheep liver histochemistry
Sheep liver for use as a positive ALP control tissue was initially frozen at -70°C before trimming into 1cm3 samples that were subsequently prepared with 0.6% Glut, 100mM TGA, or 100mM/20mM TGA/MABP for 48 hrs at room temperature. An unfixed portion was also trimmed as a control. Tissues were imbedded in OCT on dry ice and described above.
2.7. Implant preparation
Fresh porcine aortic valve leaflets and porcine aortic wall segments were shipped overnight on ice from St. Jude Medical (St. Paul, MN). On arrival, all were rinsed extensively in sterile normal saline solution, and fixed with Glut, TGA, or TGA/MABP as follows. Glut-fixed tissues were prepared as previously published [18] with 0.6% Glut (EM grade; Polysciences, Inc., Warrington, PA) in 50 mmol/L HEPES/0.9% NaCl Buffer, pH 7.4, at room temperature for 7 days with one change, then stored in 0.2% buffered Glut at room temperature until use. Tissues fixed with TGA were prepared with 100 mmol/L TGA in borate mannitol buffer (25mmol/L sodium tetraborate decahydrate, adjusted with mannitol to pH 7.4) at room temperature with daily changes of the TGA solution for 7 days. To terminate TGA crosslinking, residual epoxy groups were neutralized by overnight incubation at 37°C with 0.1 mol/L sodium thiosulfate in borate mannitol buffer (pH=7.4). For MABP addition, TGA-pretreated implants were seqentially incubated in 20mM MABP in borate mannitol buffer (pH=7.4) overnight at room temperature; residual epoxy groups were then neutralized by subsequent overnight incubation at 37°C with 0.1 mol/L sodium thiosulfate in borate mannitol buffer (pH=7.4).
An additional group was prepared by concurrent treatment with 100mM TGA and 20mM MABP over 7 days as previously described [11] prior to neutralization with sodium thiosulfate. Before implantation, all materials were rinsed three times for 1 hour in an excess of sterile normal saline, and stored at 4°C.
2.8. Rat subdermal implants
Male (21 to 25 days old) Sprague-Dawley rats were subjected to subdermal implants of fixed heterograft materials (prepared as described above) under general anesthesia (isoflurane) as previously published [18], and as approved by the Institutional Committee for the Use and Care of Animals of The Children’s Hospital of Philadelphia. The implants were either porcine aortic cusps or 1-cm2 portions of porcine aortic wall prepared as described above. Each animal was implanted with either two porcine aortic cusps or two porcine aortic wall specimens from each fixation group, in groups of 9 animals per time point per material (n=18 implants per group). Animals were sacrificed at 3, 14, 21, and 90 days after implantation and implants retrieved and rinsed in sterile saline. Explants from 3 rats per group were stripped of their fibrous capsules, and both explant and capsules stored at 4°C for later processing for Ca analyses (n=6 samples per groups). The remaining 12 explants per group were cut in half, and half of each either frozen on dry ice in OCT compound or placed in 10% neutral buffered formalin for later histological processing. Animals were weighed both at implantation and at sacrifice.
2.8.1. Explant Investigations: Morphology and Ca Analyses by Atomic Absorption Spectroscopy
Explanted samples for quantitative calcium analyses were processed by acid hydrolysis, and Ca content determined using a Perkin-Elmer model 2380 atomic absorption spectrometer (Perkin-Elmer, Inc.), as previously described [18].
2.8.2. Histological Processing of Explants
Rat subdermal explants (either fresh or formalin fixed) embedded in frozen OCT compound or paraffin were cut to 5-, Movat’s pentachrome [20], or with BCIP-NBT for ALP (as described above). Visual scoring of staining was performed in a blinded manner, assigning a numerical rating of 1 to 5 based on the following criterion: 1 = negative, 2 = rare detection, 3 = sparse but consistent, 4 = uniformly present, and 5 = intense and widespread staining.
2.9. Statistical analysis
Data for all experiments were expressed as means ± SEM (SE). The significance of differences was assessed using regression analysis between trend lines, ANOVA or Mann-Whitney rank sum test for sample sets failing a normal distribution test, or Student’s t-test or as appropriate, and was termed significant when P < 0.05. Data analysis was performed with SigmaStat for Windows 2.0.1 (SPSS Inc., Chicago, IL)
3. Results
3.1 Model system studies demonstrating reduction of alkaline phosphatase activity due to TGA-MABP under acellular conditions with two different TGA-MABP formulations
TGA-MABP crosslinked collagen substrate profoundly inhibits ALP activity (Figure 1). Furthermore, the sequential protocol for crosslinking collagen with TGA first followed by a terminal MABP exposure resulted in the greatest inhibition of ALP in these cell free studies (Fig. 1; p<0.001). TGA-pretreated collagen demonstrated the highest absorbance values for respective ALP concentrations.
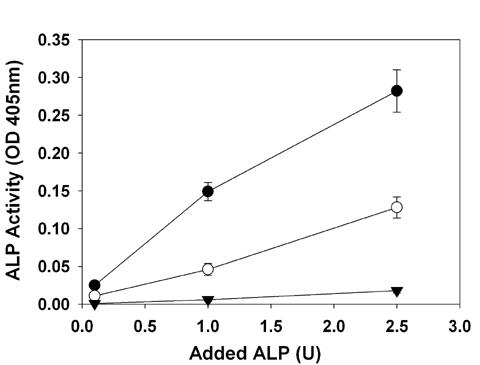
Figure 1Soluble alkaline phosphatase (ALP) activity is inhibited by MABP covalently attached to TGA-fixed collagen: the linear relationship between ALP concentration (U) and activity observed over a TGA crosslinked substrate (closed circles) was significantly reduced by concurrent covalent linkage of MABP (open circles, p<0.001) and further depressed by sequential linkage of MABP (closed triangles, p<0.001).
3.2 In vivo comparisons of TGA crosslinking with MABP vs. TGA crosslinking with a terminal MABP exposure: Porcine aortic wall--rat subdermal implant results
These investigations sought to learn if there was a difference in anticalcification efficacy in vivo between the two methods of MABP pretreatment, especially since terminal exposure results in markedly greater inhibition of alkaline phosphatase in cell free studies (Figure 1). Therefore, a set of aortic wall samples was prepared, since this type of bioprosthetic heart valve material demonstrates no inhibition of calcification following TGA exposure [11]. Rat subdermal implants were carried out for 90 days. Explant analyses revealed severe calcification of the Glut pretreated aortic wall controls (Ca=110.4± 4.06) as previously reported [10,11], but demonstrated that both TGA-MABP pretreatment protocols (with crosslinking, Ca=2.7± 0.98 & after crosslinking, Ca=3.5± 1.83) resulted in complete inhibition of calcification (p<0.001). No significant differences in calcification between the two modes of MABP addition were noted. No significant differences in rat weight gains were noted between the groups (data not shown), indicating no adverse systemic MABP events. Based on the results of these studies a decision was made to investigate only terminal exposure to MABP in the following MABP studies.
3.3 Crosslinking comparisons: Alkaline phosphatase activity in sheep liver sections is most strongly suppressed following TGA-MABP pretreatment
Liver is one of several organs where high levels of ALP are noted [21]. Comparisons of ALP staining were made before and after the crosslinking of sheep liver sections in the following stabilizing solutions: Glut, TGA, and TGA/MABP (Fig. 2). Sheep liver control sections (Fig. 2A) were intensely stained for ALP, but differed in appearance from both TGA (Fig. 2B) and Glut-prepared (Fig. 2C) sections. Both TGA and Glut sections were strongly positive, but coloration shifted from purple to blue indicating less intensive ALP activity. The TGA/MABP-prepared sections (Fig. 2D) possessed a marked reduction in ALP staining intensity.
Figure 2.
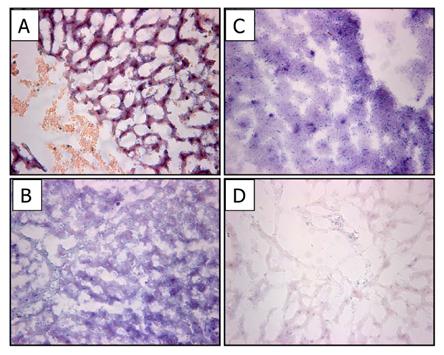
Alkaline Phosphatase activity (purple/blue BCIP/NBT staining) in sheep liver after crosslinking. (A) Frozen, non-crosslinked liver shows intense purple staining; TGA or Glut crosslinked liver (B&C respectively) display intense blue ALP staining; (D) reduction of alkaline phosphatase activity is evident in liver crosslinked with TGA/MABP. 100x Magnification.
3.4 Crosslinking comparisons: Alkaline Phosphatase activity in preimplanted cusp and wall Sections
In Glut-prepared cusp and pericardium for bioprosthetic valve applications, some low level endogenous ALP has been demonstrated [22,23]. To understand the effect of TGA crosslinking on endogenous ALP activity in porcine cusp and porcine aortic wall, sections were examined for ALP activity as with the liver tissue mentioned above. Native porcine aortic cusp demonstrates rare diffuse low-level staining (Fig. 3A). Following preparation with TGA/MABP (Fig. 3B), the cusp has lost the sparse ALP background. The same sets of studies were carried out for porcine aortic wall. Control (native) porcine aortic wall showed widely distributed cell-oriented ALP activity, and strong staining in sectioned vessels of the vasa vasorum (Fig. 3C). With TGA/MABP treatment, these microvessels remain apparently unchanged with persistent staining, but the cell-oriented staining was eradicated (Fig. 3D).
Figure 3.
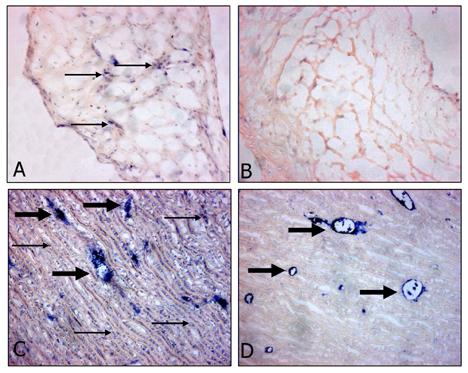
Alkaline Phosphatase activity (purple/blue color) in porcine aortic cusp (A,B) 200x magnification and porcine aortic wall (C,D) 100x magnification. (A,C) Native material, (B,D) TGA/MABP-prepared material, pre-implantation. Small arrows indicate representative regions of background alkaline phosphatase activity; large arrows indicate representative sectioned vessels of the vasa vasorum, which also stained positive. Native cuspal staining is rare compared to native aortic wall, where cell-oriented staining is widespread, but after TGA/MABP treatment is retained only by lumen of microvessels in aortic wall cross section.
3.5 Human aortic valve interstitial cell cultures: Alkaline phosphatase results from TGA/MABP crosslinked substrates
Human aortic valve interstitial cells (HAVICs) were grown on control (collagen), TGA-prepared collagen, and TGA/MABP-prepared collagen gels (Fig. 4). HAVICs exhibited similar ALP staining on collagen and TGA/collagen substrates per observation and morphometry studies (Fig. 4), with no statistically significant difference (Fig, 4D; p=0.417). However, on TGA/MABP/collagen, the mean value for ALP scoring was significantly lower than the other two preparations (p<0.001). Morphologically, HAVICs grown on untreated collagen form cellular condensations (Fig. 4A), that are different from the confluent morphology that HAVICs adopt on TGA/collagen (Fig. 4B) as previously reported using sheep AVICS [10]; condensations occur to a lesser extent on TGA/MABP substrates (Fig. 4C).
Figure 4.
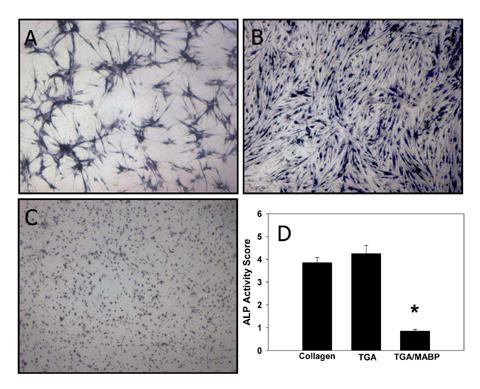
Cellular Alkaline Phosphatase activity attenuated by growth on TGA/MABP-treated collagen gel. HAVIC stained for ALP activity (BCIP/NBT; purple/blue color) after growth on (A) collagen, (B) TGA-treated collagen, or (C) TGA/MABP-treated collagen. Magnification 40x. (D) Quantification of BCIP/NBT staining (*p<0.001).
3.6 Anticalcification efficacy of TGA/MABP: rat subdermal implant time course results
Explant time points were at 3, 14, 21, and 90 days. The foreign body response was observed to involve the encapsulation of implanted materials in a fibrous capsule. Upon explantation, capsules were separated from respective implants and both materials’ calcium content was determined independently through atomic absorption spectroscopy.
General trends include the observation that Glut-prepared cusp had greater levels of calcification than similarly prepared aortic wall (Figure 5) and both types of implants progressively calcified to levels which exceeded 160±8 (P<0.001). The reverse was seen for preparation with TGA-pretreated aortic wall, which also calcified severely, reaching 143±11 μg/mg, vs. TGA-pretreated cusp which resisted significant calcification until 21 days (p<0.001) and thereafter did not exceed calcium levels of 35±11 μg/mg. After pretreating with TGA/MABP, both materials (cusp and aortic wall) had calcium levels that never increased over 8±2 μg/mg during the time course of the study, with some statistical variation at timepoints 14 and 90 days in the two groups respectively (p=0.012 and 0.041).
Figure 5.
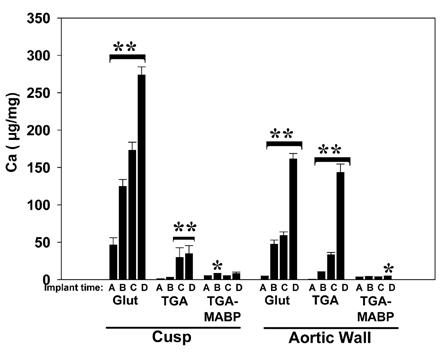
Timecourse of calcification in aortic wall or cusp rat subdermal implants. Calcium was measured after explantation at: A= 3 days, B= 14 days, C= 21 days, or D= 90 days, of implant materials crosslinked with Glut, TGA, or TGA/MABP. Glut-prepared materials calcified severely, even by 14 days (p<0.001). TGA is protective for cusp compared to Glut (p<0.001 at all timepoints; cusp resisted significant calcification at 14 days), but not aortic wall. TGA/MABP explants remained essentially free from calcium deposition at 90 days, with statistically significant variations in the calcium content of explanted cusp at 14 days, and aortic wall at 90 days. **p<0.001 increases over timecourse within bracket; *p<0.01 vs control only.
3.6.1 Fibrous capsule analyses
Capsule calcium levels (Figure 6) were an order of magnitude or more below the cuspal and aortic wall explant samples. While there was an apparent trend for increased capsular calcium in both Glut and TGA groups, only the 90-day TGA-cusp group was significantly (p<0.01) elevated compared to earlier timepoints. Capsules analyzed from TGA-MABP pretreated porcine aortic cusp and aortic wall demonstrated no significant changes in calcium content over the time course of the study. There was no correlation between capsular and explant calcium values in any of the groups (data not shown).
Figure 6.
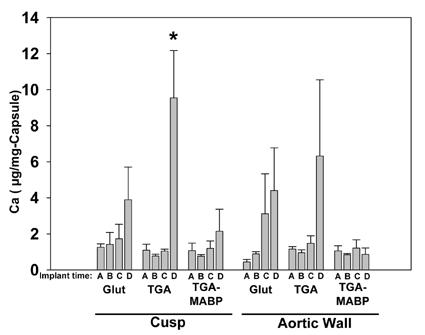
Calcium concentrations in the fibrous capsules removed from porcine aortic wall sections and porcine aortic cusp sections explanted at timepoints A= 3 days, B= 14 days, C= 21 days, and D= 90 days. Respective preparations of explants were with Glut, TGA, and TGA/MABP. Capsules showed low levels of calcification compared to the explants (see Fig. 6), and with the exception of capsules from 90-day TGA cusp explants, no statistically significant increases over time (*p<0.01).
3.7 In Situ (with surrounding fibrous capsule) Explant Histochemistry/Immunostaining
Explants from all time points (3 day, 14 day, 21 day, 90 day) and preparations (Glut, TGA, TGA/MABP) were prepared for histochemical analyses for ALP, Alizarin Red S, and Movat’s pentachrome. For these explants, care was taken to maintain the integrity of the anatomic capsule/explant relationship. At day 3, minimal fibrous capsular formation can be observed (data not shown), and is fully developed by day 14 (data not shown). Since our interest lies primarily in understanding long-term changes in heterograft materials, the results presented show only the longest (90 day) time point.
3.7.1 Alizarin Red Histochemistry
Neither cusp nor aortic wall prepared with TGA/MABP had evidence of calcification as indicated by Alizarin Red at any of the time points (Figure 7), in agreement with the chemical analyses (Fig. 5). Glut and TGA preparation of both cusp and aortic wall appeared similar in that there was a high degree of red staining indicating calcification in the explants, yet little to no calcification present in the capsular material (Figs. 7A&D, B&E respectively). Visual scoring of the degree of staining (Figure 7G) also paralleled chemical quantification, with significant increases over time in all groups (p<0.001) except the TGA/MABP pretreated materials. TGA/MABP cusp and aortic wall had Alizarin scoring (Figure 7G) consistently <1 (range = 0.0 - 0.3) for all time points, and while there was minor variation within the TGA/MABP pretreated cusp group (day 21 was statistically higher than day 3: 0±0 vs. 0.09±0.02 respectively, p<0.001), there was no significant difference at any timepoint within the TGA/MABP pretreated aortic wall group.
Figure 7.
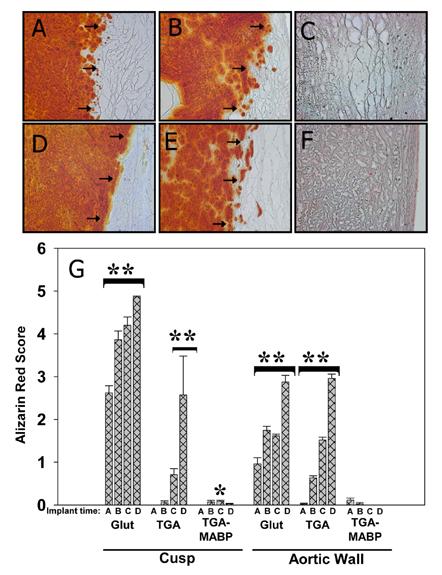
Alizarin Red staining of 90 day explanted aortic cusp (A:Glut, B:TGA, and C:TGA/MABP) and aortic wall (D:Glut, E:TGA, and F:TGA/MABP). Representative examples of explants with accompanying capsule, showing distribution of calcification (red color) in tissues. Arrows indicate boundary between explant (left), and fibrous capsule (right) formed as a result of host response. While Glut or TGA-prepared materials are heavily calcified at 90 days, TGA/MABP-prepared materials are calcium free. Little or no calcification is seen in any of the accompanying capsular material. Magnification 200x. (G) Alizarin Red histochemistry scoring results from sectioned explants. Glut-prepared specimens had visible calcium deposition that increased over the 90 day period. Specimens prepared solely with TGA similarly increased Alizarin staining, with the exception that significant increases were delayed until 21 days in cusp. No significant calcification was seen in TGA/MABP-pretreated aortic wall explants, and statistically significant variations in calcium phosphate staining were only seen at day 21 in TGA/MABP pretreated cusps. **p<0.001 increases over timecourse within bracket; *p<0.001 vs Glut control only.
3.7.2 Alkaline Phosphatase Histochemistry of In Situ Explant-Fibrous Capsule Specimens
Alkaline phosphatase staining (BCIP, per Methods) in general revealed positive staining only in the fibrous capsule (Figure 8), regardless of pretreatment, which increased over time (data not shown). Furthermore, BCIP staining of cusp and aortic wall implants was sparse and did not differ significantly from that noted in unimplanted samples (Figure 3). Thus, these results indicate alkaline phosphatase activity that could contribute to phosphoester hydrolysis in proximity to the subdermal implants is emanating from the fibrous capsule and not from either endogenous enzyme or alkaline phosphatase adsorbed to the implants.
Figure 8.
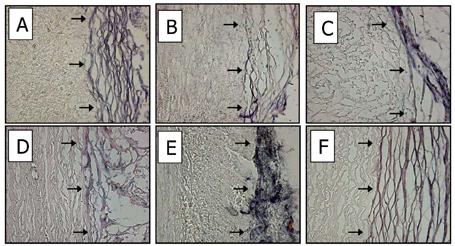
ALP staining of 90 day aortic cusp (A:Glut, B:TGA, and C:TGA/MABP) and 90 day aortic wall (D:Glut, E:TGA, and F:TGA/MABP); representative explants with accompanying capsule, showing distribution of ALP activity (purple/blue color) in tissues. Arrows indicate boundary between explant (left), and fibrous capsule (right) formed as a result of host response. In all cases, the capsule is positive for alkaline phosphatase activity using the BCIP stain for alkaline phosphatase activity. Magnification 200x .
3.7.3 Movat’s histochemistry staining of TGA-MABP pretreated explants
Although TGA/MABP pretreated implants did not calcify, Movat’s staining studies were carried out to learn if, despite the absence of pathologic calcification, there had been structural deterioration of TGA/MABP porcine aortic cusp and aortic wall as a result of 90 days subdermal implantation. Movat’s pentachrome stains for various tissue constituents: black, elastin; yellow, collagen; blue, proteoglycans; and red for fibrin and muscle. Cusp is primarily comprised of proteoglycans, collagen, and some elastic fibers. Both unimplanted control (Figure 9A) and explant (Figure 9B) photos show the abundance of these materials as evidenced through Movat’s staining (yellow, blue, and black). The capsule/implant boundary in the 90 day explants show no changes in the tissue architecture of the implant. In Figs. 9C & 9D, aortic wall is shown comparing unimplanted, and 90 day explants, respectively, format. In aortic wall, there is a strong presence of elastic fibers that is unchanged in the 90 day explant. Nevertheless, in both aortic wall and cusp, the capsule attaches to the surface of the implant in a smooth transition with minimal to no change in the outermost layer of the implant. In summary, little to no change in tissue architecture in TGA-MABP crosslinked, noncalcified explants is evident.
Figure 9.
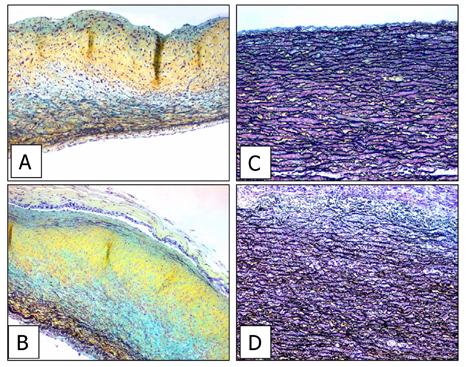
Movat’s pentachrome staining of crosslinked porcine aortic cusp (A & B) and porcine aortic wall (C & D). Panels A &C: unimplanted TGA/MABP-prepared controls. Panels B &D: subcutaneous 90 day explants, TGA/MABP-pretreated. Neither calcific deposits nor gross structural changes are seen in panels B & D. Black coloration indicates elastic fibers, yellow is collagen, blue is proteoglycans, and red indicates fibrin and muscle. Magnification 50x.
4. Discussion
The present paper reports a detailed efficacy and mechanism study of the bisphosphonate, MABP, used in conjunction with TGA crosslinking of porcine aortic valve bioprosthetic cusp and aortic wall. The results of these investigations are of particular significance in that they have strongly confirmed the efficacy of MABP for completely eliminating bioprosthetic aortic cusp and wall calcification in long term rat subdermal implants. Furthermore, our preliminary report [11] involved only using MABP at the same time as TGA crosslinking was taking place, thereby potentially compromising the integrity of the crosslinking process. The present studies demonstrate the novel finding that the terminal use of MABP after TGA crosslinking is complete has the same profound anticalcification efficacy as MABP used at the same time as TGA crosslinking, and in fact has more significant ALP inhibitory effects in mechanistic studies reported herein. Furthermore, this paper also reports the unique observation that an immobilized bisphosphonate, MABP, result in reduced ALP activity both in terms of its effects on free enzyme and cellular based ALP activity.
Our initial TGA studies [10] reported the synthesis of this polyepoxide crosslinker and its efficacy in 21 day rat subdermal implants. Mechanistic studies indicated that TGA crosslinking altered extra cellular matrix signaling activity, and this may be in part responsible for the inhibition of calcification noted with TGA used alone. Our preliminary report investigating longer termed implants showed that despite TGA crosslinking, breakthrough calcification could be noted with bioprosthetic leaflet materials. In addition, this preliminary report also noted that TGA used alone had no effect on inhibiting porcine aortic wall calcification. Thus, a thiol-bisphosphonate, MABP, was synthesized and characterized [11]. Initial investigations revealed that when MABP was used as a pretreatment in combination with TGA crosslinking, there was complete inhibition of calcification of bioprosthetic leaflet and aortic wall calcification in rat subdermal implants, although it was observed that concentrations of MABP greater than 20mM caused unquestionable interference with TGA crosslinking [11]. Thus, this provided the impetus for the present studies, which investigated the hypothesis that there would be sufficient residual epoxy activity available following TGA crosslinking to reactively bind MABP through thiol-epoxy reactions. The results presented in this study support this hypothesis.
Previous studies by others have investigated poly-epoxy compounds for crosslinking bioprosthetic heart valves, with no definitive efficacy reported for preventing calcification [22-25]. This may have been due to the fact that these investigations were carried out with alkyl polyepoxides [22-25], rather than highly polar polyepoxides, such as TGA that was investigated in these studies. Alkyl-epoxides can have poor water solubility characteristics, and frequently require the use of organic co-solvents in order to obtain even partially aqueous solutions. TGA by comparison is highly water soluble [10], and thus should hypothetically have better bioprosthetic tissue penetration and biocompatibility characteristics. This was certainly born out by our initial studies [10].
The present studies also moved beyond our preliminary report that [11] described the synthesis and early efficacy observations for MABP use with TGA crosslinking of bioprosthetic materials, in that the ALP interactions of MABP have now been carefully examined. The results were reported herein strongly support the hypothesis that MABP inhibition of ALP may strongly contribute to the anti-calcification efficacy of TGA crosslinked bioprosthetic materials pretreated with MABP as a terminal procedure. At each level examined, isolated enzyme inhibition and inhibition of cellular generated ALP, MABP’s specific interactions that overall reduce ALP activity are very apparent.
Although previous observations [26,27] have indicated that ALP can remain active in bioprosthetic heart valve leaflets and aortic wall even after Glut crosslinking, our studies examining this phenomenon indicated that this level of ALP activity after either TGA or Glut crosslinking is in fact sparse. This was the basis for the rationale for the positive control studies carried out with TGA and TGA-MABP crosslinking of liver as an ALP enriched positive control tissue, with profound inhibition of ALP by TGA immobilized MABP. Along these lines our studies also indicated the strong presence of ALP activity in the fibrous capsule that surrounds subdermal implants, an observation not reported previously by either our group or others. This observation suggests that the fibrous capsule in fact provides ALP in proximity to the implants thereby facilitating the hypothetical creation of inorganic phosphate from the hydrolysis of phosphoesters. Although fibrous capsules do not exist in blood stream implants of bioprosthetic heart valves, serum typically contains high levels of ALP, and this may serve a comparable function in terms of providing an ALP source comparable to that observed in these studies with respect to the fibrous capsule. It is also interesting to note that there was very low-level calcification of the fibrous capsule over time compared to the bioprosthetic leaflet and aortic wall materials. The reasons for this are not clear, but may most likely be explained by the fact that the fibrous capsule is a viable, actively remodeling connective tissue matrix, in contrast to the chemically crosslinked non-viable bioprosthetic leaflet and cusp, which are highly susceptible to calcification due to the inability to de-process calcium entry into potential calcification sites and the presence of associated inorganic phosphate due to ALP activity, thus leading to calcium phosphate crystal formations.
Thus, the results of the present studies overall demonstrate that TGA crosslinking followed by MABP exposure provides significant anticalcification efficacy. However, the present subdermal studies need to be confirmed in experimental circulatory implants, such as sheep mitral valve replacements, to confirm that the anticalcification mechanisms described herein are also active and effective in the blood-material environment. Similarly, the morphologic stability observed in the present TGA/MABP subdermal explants needs to be assessed further in the more biomechanically challenging environment of an actual experimental heart valve replacement.
5. Conclusions
These studies have demonstrated the efficacy of MABP pretreatment used following optimal TGA crosslinking as a definitive means of imparting optimal anti-calcification efficacy for porcine aortic bioprosthetic cusp and aortic wall in 90 day rat subdermal implant studies. The mechanism of action of covalently attached MABP would seem to be associated with reduction in ALP activity, that is due to direct inhibition of ALP at the enzymatic level and inhibition of cell associated ALP. Furthermore, TGA crosslinking with the terminal use of MABP not only significantly inhibits calcification, but also results in the apparent morphologic stability of the valve leaflet and aortic wall structure per histology results.
Acknowledgement
The authors thank Ms. Jennifer LeBold for her help in processing this manuscript. This research was supported in parts by grants from the National Heart, Lung and Blood Institute (P50-HL74731, T32-HL7915), a grant from St. Jude Medical, Inc., and the William J. Rashkind Endowment of the Children’s Hospital of Philadelphia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore MA, Chen WM, Phillips RE, Bohachevsky IK, McIlroy BK. Shrinkage temperature versus protein extraction as a measure of stabilization of photooxidized tissue. J Biomed Mater Res. 1996;32:209–14. doi: 10.1002/(SICI)1097-4636(199610)32:2<209::AID-JBM9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 2.Lerones C, Mariscal A, Carnero M, Garcia-Rodriguez A, Fernandez-Crehuet J. Assessing the residual antibacterial activity of clinical materials disinfected with glutaraldehyde, o-phthalaldehyde, hydrogen peroxide or 2-bromo-2-nitro-1,3-propanediol by means of a bacterial toxicity assay. Clin Microbiol Infect. 2004;10:984–9. doi: 10.1111/j.1469-0691.2004.00967.x. [DOI] [PubMed] [Google Scholar]
- 3.Chang Y, Hsu CK, Wei HJ, Chen SC, Liang HC, Lai PH, Sung HW. Cell-free xenogenic vascular grafts fixed with glutaraldehyde or genipin: in vitro and in vivo studies. J Biotechnol. 2005;120:207–19. doi: 10.1016/j.jbiotec.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Mirzaie M, Meyer T, Saalmuller A, Dalichau H. Influence of glutaraldehyde fixation on the detection of SLA-I and II antigens and calcification tendency in porcine cardiac tissue. Scand Cardiovasc J. 2000;34:589–92. doi: 10.1080/140174300750064530. [DOI] [PubMed] [Google Scholar]
- 5.Dumont CE, Plissonnier D, Guettier C, Michel JB. Effects of glutaraldehyde on experimental arterial iso- and allografts in rats. J Surg Res. 1993;54:61–9. doi: 10.1006/jsre.1993.1011. [DOI] [PubMed] [Google Scholar]
- 6.Webb CL, Schoen FJ, Levy RJ. Covalent binding of aminopropanehydroxydiphosphonate to glutaraldehyde residues in pericardial bioprosthetic tissue: stability and calcification inhibition studies. Exp Mol Pathol. 1989;50:291–302. doi: 10.1016/0014-4800(89)90039-7. [DOI] [PubMed] [Google Scholar]
- 7.Huang-Lee LL, Cheung DT, Nimni ME. Biochemical changes and cytotoxicity associated with the degradation of polymeric glutaraldehyde derived crosslinks. J Biomed Mater Res. 1990;24:1185–201. doi: 10.1002/jbm.820240905. [DOI] [PubMed] [Google Scholar]
- 8.Simmons DM, Kearney JN. Evaluation of collagen cross-linking techniques for the stabilization of tissue matrices. Biotechnol Appl Biochem. 1993;17:23–9. [PubMed] [Google Scholar]
- 9.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–80. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Connolly JM, Alferiev I, Clark-Gruel JN, Eidelman N, Sacks M, Palmatory E, Kronsteiner A, Defelice S, Xu J, Ohri R, Narula N, Vyavahare N, Levy RJ. Triglycidylamine crosslinking of porcine aortic valve cusps or bovine pericardium results in improved biocompatibility, biomechanics, and calcification resistance: chemical and biological mechanisms. Am J Pathol. 2005;166:1–13. doi: 10.1016/S0002-9440(10)62227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alferiev IS, Connolly JM, Levy RJ. A novel mercapto-bisphosphonate as an efficient anticalcification agent for bioprosthetic tissues. J Organometallic Chem. 2005;690:2543–2547. [Google Scholar]
- 12.Reszka AA, Rodan GA. Mechanism of action of bisphosphonates. Curr Osteoporos Rep. 2003;1:45–52. doi: 10.1007/s11914-003-0008-5. [DOI] [PubMed] [Google Scholar]
- 13.Vaisman DN, McCarthy AD, Cortizo AM. Bone-specific alkaline phosphatase activity is inhibited by bisphosphonates: role of divalent cations. Biol Trace Elem Res. 2005;104:131–40. doi: 10.1385/BTER:104:2:131. [DOI] [PubMed] [Google Scholar]
- 14.Alferiev I, Vyavahare N, Song C, Connolly J, Hinson JT, Lu Z, Tallapragada S, Bianco R, Levy R. Bisphosphonate derivatized polyurethanes resist calcification. Biomaterials. 2001;22:2683–93. doi: 10.1016/s0142-9612(01)00010-2. [DOI] [PubMed] [Google Scholar]
- 15.Webb CL, Benedict JJ, Schoen FJ, Linden JA, Levy RJ. Inhibition of bioprosthetic heart valve calcification with aminodiphosphonate covalently bound to residual aldehyde groups. Ann Thorac Surg. 1988;46:309–16. doi: 10.1016/s0003-4975(10)65932-2. [DOI] [PubMed] [Google Scholar]
- 16.Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75:457–65. doi: 10.1016/s0003-4975(02)04312-6. [DOI] [PubMed] [Google Scholar]
- 17.Fishbein I, Alferiev IS, Nyanguile O, Gaster R, Vohs JM, Wong GS, Felderman H, Chen IW, Choi H, Wilensky RL, Levy RJ. Bisphosphonate-mediated gene vector delivery from the metal surfaces of stents. Proc. Natl. Acad. Sci. USA. 2006;103:159–64. doi: 10.1073/pnas.0502945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vyavahare N, Hirsch D, Lerner E, Baskin JZ, Schoen FJ, Bianco R, Kruth HS, Zand R, Levy RJ. Prevention of bioprosthetic heart valve calcification by ethanol preincubation. Efficacy and mechanisms. Circulation. 1997;95:479–488. doi: 10.1161/01.cir.95.2.479. [DOI] [PubMed] [Google Scholar]
- 19.Vyavahare N, Jones PL, Tallapragada S, Levy RJ. Inhibition of matrix metalloproteinase activity attenuates tenascin-C production and calcification of implanted purified elastin in rats. Am J Pathol. 2000;157:885–893. doi: 10.1016/S0002-9440(10)64602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell HK. A modification of the Movat pentachrome stain. Arch Pathol. 1972;94:187. [PubMed] [Google Scholar]
- 21.Henthorn PS, Raducha M, Edwards YH, Weiss MJ, Slaughter C, Lafferty MA, Harris H. Nucleotide and amino acid sequences of human intestinal alkaline phosphatase: close homology to placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1987;84(5):1234–8. doi: 10.1073/pnas.84.5.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imamura E, Sawatani O, Koyanagi H, Noishiki Y, Miyata T. Epoxy compounds as a new cross-linking agent for porcine aortic leaflets: subcutaneous implant studies in rats. J Card Surg. 1989;4:50–7. doi: 10.1111/j.1540-8191.1989.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 23.Xi T, Ma J, Tian W, Lei X, Long S, Xi B. Prevention of tissue calcification on bioprosthetic heart valve by using epoxy compounds: a study of calcification tests in vitro and in vivo. J Biomed Mater Res. 1992;26:1241–51. doi: 10.1002/jbm.820260913. [DOI] [PubMed] [Google Scholar]
- 24.Sung HW, Shen SH, Tu R, Lin D, Hata C, Noishiki Y, Tomizawa Y, Quijano RC. Comparison of the cross-linking characteristics of porcine heart valves fixed with glutaraldehyde or epoxy compounds. Asaio J. 1993;39:532–6. [PubMed] [Google Scholar]
- 25.Shen SH, Sung HW, Tu R, Hata C, Lin D, Noishiki Y, Quijano RC. Characterization of a polyepoxy compound fixed porcine heart valve bioprosthesis. J Appl Biomater. 1994;5:159–62. doi: 10.1002/jab.770050209. [DOI] [PubMed] [Google Scholar]
- 26.Maranto AR, Schoen FJ. Alkaline phosphatase activity of glutaraldehyde-treated bovine pericardium used in bioprosthetic cardiac valves. Circ Res. 1988;63:844–8. doi: 10.1161/01.res.63.4.844. [DOI] [PubMed] [Google Scholar]
- 27.Maranto AR, Schoen FJ. Phosphatase enzyme activity is retained in glutaraldehyde treated bioprosthetic heart valves. ASAIO Trans. 1988;34:827–30. [PubMed] [Google Scholar]


