Abstract
The results of administering escalating, i.v. doses of targeted nanoparticles containing a siRNA targeting the M2 subunit of ribonucleotide reductase to non-human primates are reported. The nanoparticles consist of a synthetic delivery system that uses a linear, cyclodextrin-containing polycation, transferrin (Tf) protein targeting ligand, and siRNA. When administered to cynomolgus monkeys at doses of 3 and 9 mg siRNA/kg, the nanoparticles are well tolerated. At 27 mg siRNA/kg, elevated levels of blood urea nitrogen and creatinine are observed that are indicative of kidney toxicity. Mild elevations in alanine amino transferase and aspartate transaminase at this dose level indicate that the liver is also affected to some extent. Analysis of complement factors does not reveal any changes that are clearly attributable to dosing with the nanoparticle formulation. Detection of increased IL-6 levels in all animals at 27 mg siRNA/kg and increased IFN-γ in one animal indicate that this high dose level produces a mild immune response. Overall, no clinical signs of toxicity clearly attributable to treatment are observed. The multiple administrations spanning a period of 17–18 days enable assessment of antibody formation against the human Tf component of the formulation. Low titers of anti-Tf antibodies are detected, but this response is not associated with any manifestations of a hypersensitivity reaction upon readministration of the targeted nanoparticle. Taken together, the data presented show that multiple, systemic doses of targeted nanoparticles containing nonchemically modified siRNA can safely be administered to non-human primates.
Keywords: systemic administration, RNA interference
A fundamental cellular mechanism for inhibiting gene expression, RNAi has quickly become a standard for drug discovery research. If therapeutics that are based on RNAi can be developed, they will have the potential to work on all classes of molecular targets, including many that are not treatable with conventional small molecules, antibodies, or other biologic agents (1). Synthetic siRNAs that are double-stranded and 21–23 nt in length use the naturally occurring RNAi process and are being explored as potential therapeutics in human clinical trials (1, 2). These trials involve localized delivery to the eye, nose, and lung (1). An important goal for siRNA-based drugs is targeted, systemic administration, and effective delivery of siRNA is likely the most challenging barrier to the widespread application of RNAi-based therapeutic use in humans.
Naked siRNA is unstable in plasma, and chemical modifications that impart in vivo stability have been used systemically in animal models (3, 4). In these studies, the siRNA doses were 30–50 mg/kg in mice. Such large doses may be contraindicated for therapeutic applications in humans because of costs and safety issues (5). However, encapsulation of the siRNA in lipid-based nanoparticles of ≈100-nm diameter provided effective dosing in mice (5, 6) and monkeys (6) at amounts ≈1–3 mg/kg. These delivery systems did not contain targeting ligands, and the siRNA was shown to accumulate in the liver where it inhibited protein expression.
Numerous studies have used ligand-targeted siRNA (7). Antibody (8) and aptamer (9, 10) targeting agents have been investigated in mouse models (8, 9). These conjugates carry <10 siRNA molecules per targeting agent. Alternatively, ligand-containing nanoparticles have been used (7) and have adjustable ligand numbers and larger siRNA payloads. For example, 70-nm nanoparticles can contain 1–100 transferrin (Tf)-targeting ligands and 2,000 siRNA molecules, yielding siRNA/targeting ligand ratios of 20:1 or higher (11). Nanoparticles containing multiple targeting ligands can have multivalent binding to cell surfaces and deliver a larger payload of siRNA than molecular conjugates.
No reports of targeted delivery of siRNA in non-human primates have appeared to our knowledge. Naked siRNA has been administered to rhesus macaques through intranasal instillation (12), and lipid-formulated siRNA was injected via the saphenous vein (single injection only) into cynomolgus monkeys (6). Numerous investigations of targeted, systemic delivery of siRNA using mouse models have been reported. Those studies raise issues of concern for the safe administration of multiple doses of targeted, systemic delivery of siRNA. Although naked siRNA is not inherently immunostimulatory (13), lipid delivery of double-stranded siRNA and single-stranded RNA can stimulate the immune systems of mammals (14–16). Lipid-based endosomal delivery of siRNA containing immunostimulatory motifs activate Toll-like receptors (TLRs), such as TLR7, TLR8, and TLR9, in peripheral blood mononuclear cells, monocytes, plasmacytoid dendritic cells, nonplasmacytoid dendritic cells (14–16), and CD34+ progenitor cells (17). Fortunately, chemically modified siRNAs can be prepared that minimize their immunostimulatory behavior (18). Interestingly, the delivery of siRNA via the cyclodextrin polycation system developed in our laboratory at the California Institute of Technology did not produce immune stimulation in mice, even when the siRNA contained a highly stimulatory base sequence motif (19). Possible reasons for the lack of immune stimulation include endosomal buffering by this delivery system (20, 21) [inhibitors of endosomal acidification have been shown to block immune activation (16)] and lack of uptake by specific immunocompetent cell populations (this system does not enter CD34+ progenitor cells; J.D.H. and J. J. Rossi, unpublished data).
Targeted, systemic delivery vehicles contain a targeting ligand, and our investigations have shown that our formulations can be sterically stabilized by PEG (11, 19). PEGylated lipid systems carrying small molecule drugs have been used clinically for years, and these lipid-based systems are capable of causing hypersensitivity reactions and complement activation (22, 23). Additionally, repeated administration of lipid-based delivery vehicles has the potential to cause phospholipidosis (24) and other lipid-related toxicities. Recently, PEGylated liposomes have been shown to have accelerated clearance from blood and can elicit acute hypersensitivity upon repeated dosing that is caused by a long-lived antibody response generated against the PEGylated particle (25–28). Thus, the safety of targeted, systemic delivery vehicles containing siRNA will require multidosing studies to properly assess their behavior.
We have been investigating a cyclodextrin polycation delivery system that is capable of targeted, systemic delivery of siRNA in mice (19). Here, we report on a multidosing study of siRNA in non-human primates with a targeted, systemic delivery system. The results of this investigation suggest that this delivery system can be safely used for repeated systemic administration of siRNA.
Results and Discussion
Nanoparticles Containing Tf-Targeting Ligand and siRNA Are Active in Mouse, Monkey, and Human Systems.
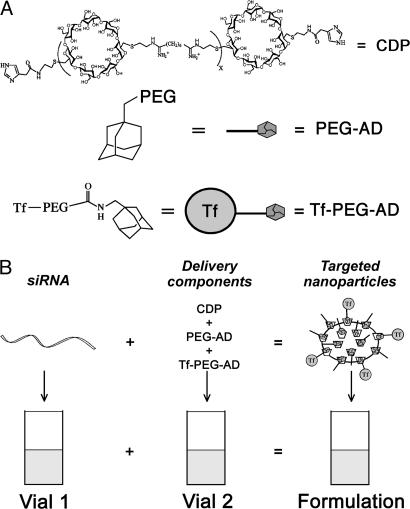
Fig. 1 schematically illustrates the delivery system and its formulation with siRNA to prepare targeted nanoparticles; these nanoparticles use human Tf as the targeting ligand. This targeted particle has been shown to reach tumors from an i.v. injection in mice (19, 29) and give sequence-specific gene inhibition that leads to antitumor efficacy (19). The Tf receptor (TfR) has long been known to be up-regulated in malignant cells (30). Tf targeting to TfR has been used for drug delivery in human clinical trials with adriamycin (31) and cisplatin (32) and is currently in a phase III trial for brain cancer with diphtheria toxin (33). Human Tf can bind to the TfR in mouse, rat and monkey [data provided in supporting information (SI) Fig. 6] and therefore provides a useful targeting agent for use in these species without a change in formulation when proceeding to dosing in humans. The particles formed are ≈70 nm in diameter (11) and have been shown to localize and penetrate throughout tumors in a metastatic mouse model of Ewing's sarcoma (19). The siRNA sequence is a potent inhibitor of the M2 subunit of ribonucleotide reductase (RRM2) (34) and is active in mouse, monkey, and human cells (ref. 34 and SI Fig. 7). The cross-species function of both the targeting ligand and the siRNA allows for studies in rodents and non-human primates by using the exact formulation proposed for use in humans.
Fig. 1.
Schematic illustration of the delivery system (A) and its formulation with siRNA (B).
Non-Human Primate Study Design.
Fig. 2 outlines the in-life study in three female cynomolgus monkeys, including dosing and blood sampling schedules. For treatment phases 2–4, dosing of animal 1 was performed 1 day ahead of the other two animals to allow a 24-h monitoring period for each escalating dose level before further animal treatment. All animals successfully received all doses shown in Fig. 2. The 27-mg/kg dose is very large for a formulated siRNA. For example, this amount is equivalent to a mouse dose (adjusted for body surface area in mg/m2) of 109 mg/kg. Typical dosing amounts in formulations of siRNA in mice (including the cyclodextrin system) are ≈1–5 mg/kg (5, 6, 19). Thus, the 27-mg/kg dose is ≈20–100 times larger than those that have shown efficacy in mouse models (when doses are extrapolated across species on a body surface area basis). After the 27-mg/kg dosing, a washout period of 11–12 days was provided to allow for the possible formation of anti-Tf antibodies. Final dosing at 3 mg/kg was performed at a time when antibodies may be present and their effects on the animal and/or siRNA pharmacokinetics (PK) could be evident.
Fig. 2.
Overview of experimental protocol. Three female, non-naïve cynomolgus monkeys were treated with an siRNA-containing nanoparticle formulation. Formulations were freshly prepared on the day of treatment and had a final siRNA concentration of 1 mg/ml. A simple slow-push i.v. injection was used to administer the 3-mg/kg (with respect to siRNA) and 9-mg/kg doses. The large volume needed for the 27-mg/kg dose required administration of this dose by 2-h i.v. infusion; the 0-mg/kg dose (D5W only) was also delivered by infusion. The 0-, 3-, 9-, and 27-mg/kg doses were administered sequentially, with 2–3 days between each dose, as indicated. After the 27-mg/kg dosing, a washout period of 11–12 days was allowed before a final 3-mg/kg dose was given. Blood samples were drawn at the indicated times for analyses of complete blood counts, serum chemistry, coagulation parameters, complement factors, antibodies, cytokines, and PK.
Clinical Signs, Body Weight, and Food Consumption.
Clinical observations considered potentially treatment-related were limited mainly to the highest dose level (27 mg/kg) and included dry or hard feces and apparent inappetence. Hard feces were observed in the cage pans of all animals upon the initial observation after the phase 4 dosing (27 mg/kg). All animals exhibited inappetence (low biscuit consumption) at the initial observation after phase 4 (27 mg/kg) dosing. This observation is consistent with the findings of a loss of body weight and decrements in food consumption, as discussed below. However, these events were not clearly treatment-related.
There was no effect on food consumption. Sporadically low biscuit consumption was not temporally related to dosing with the test material and was attributed to inappetance caused by excitement or stress of the study animals because of the intensive study procedures (i.e., the repeated restraint for dosing, activity in the animal room, and the intensive blood sampling schedule). A slight decrease in body weight occurred in the animals over the course of the study, but this change was considered secondary to the inappetance that was most likely stress-related.
Assessment of Hematology, Serum Chemistry, Coagulation Parameters, Complement Parameters, Antibodies, and Cytokines.
Blood samples were collected at the times indicated in Fig. 2 and used to assess hematology, serum chemistry, coagulation, complement activation, immune and antibody responses, and PK. All hematological parameters remained within the range of the predosing values and the normal ranges for monkeys, except two animals had slightly elevated mean platelet volume (11.1 and 11.9 fl, compared with 8.0 fl prestudy mean) and lowered platelet counts (196 and 280 × 103/μl, vs. 549 × 103/μl prestudy mean) 24 h after receiving the 27-mg/kg dose.
Serum chemistry values all remained within range of the predosing values except for aspartate transaminase (AST), alanine amino transferase (ALT), blood urea nitrogen (BUN), and creatinine (CRE) 24 h after receiving the 27-mg/kg dose (Table 1). The increases in BUN and CRE are indicative of toxicity to the kidney. Because the formulation without siRNA was not tested in this study, it is uncertain whether the apparent renal toxicity was caused by the siRNA or one or more of the formulation constituents. It is unlikely that the kidney toxicity is related to the siRNA based on unpublished experience with i.v. injection of large doses of naked siRNAs. The mild increases in AST and ALT (≈2-fold, relative to prestudy) suggest that there may be some effect on the liver at the highest dose level. However, the increases are well below the range that would be associated with significant hepatotoxicity.
Table 1.
Serum chemistry and coagulation analyses
| Treatment | AST, units/liter | ALT, units/liter | BUN, mg/dl | CRE, mg/dl | PT, s | APTT, s | Fibrinogen, mg/dl |
|---|---|---|---|---|---|---|---|
| Acclimation | 31 ± 6 | 38 ± 24 | 16 ± 6 | 0.8 ± 0.2 | 10.0 ± 0.2 | 18.7 ± 1.7 | 296 ± 63 |
| D5W | 33 ± 6 | 33 ± 8 | 15 ± 5 | 0.8 ± 0.1 | 11.0 ± 0.1 | 18.1 ± 1.1 | 286 ± 31 |
| 3 mg/kg | * | * | 13 ± 3 | 0.7 ± 0.1 | 11.5 ± 1.2 | 21.3 ± 1.9 | 289 ± 77 |
| 9 mg/kg | 41 ± 7 | 88 ± 71 | 12 ± 3 | 0.8 ± 0.1 | 10.4 ± 0.4 | 21.5 ± 2.5 | 230 ± 22 |
| 27 mg/kg | 79 ± 26 | 90 ± 26 | 29 ± 9 | 4.0 ± 2.3 | 12.6 ± 3.6 | 23.3 ± 2.9 | 197 ± 11 |
Data were collected 24 h after each dose. PT, prothrombin time; APTT, activated partial thromboplastin time.
*At this dose level, one animal gave elevated levels that were not observed upon subsequent higher doses. Animal 1: AST = 27 units/liter, ALT = 24 units/liter; animal 2: AST = 39 units/liter, ALT = 55 units/liter; animal 3: AST = 534 units/liter, ALT = 600 units/liter.
Coagulation parameters (prothrombin time, activated partial thromboplastin time, and fibrinogen) were not substantially affected by the targeted nanoparticles, even at the highest dose level tested (Table 1). Prothrombin time was very slightly prolonged in one animal on day 11, activated partial thromboplastin time was also slightly prolonged in all animals on days 10/11 (1 day after the 27-mg/kg dose), and fibrinogen concentration was slightly decreased in all animals on the same day. However, these changes were small in magnitude and did not deviate from the normal ranges for monkeys; hence, they could not be clearly distinguished from normal variation.
Overall, the evaluation of hematological, coagulation, and serum chemistry parameters indicated that the targeted nanoparticles were well tolerated, except at the 27-mg/kg dosing level where there were some indications of an impact on the animals. The most noteworthy change was the increase in BUN and CRE indicative of renal dysfunction.
Table 2 lists data that show there was no complement activation by the targeted nanoparticles. Neither Bb nor CH50 exhibited significant changes at any dose level, relative to predose values, suggesting that both the classical and alternative complement pathways were not highly activated. The slight reduction in mean CH50 5 min after the 27-mg/kg dose (relative to the predose mean) was within the range of normal variation. Although an effect of the 27-mg/kg dose could not be ruled out, this difference was considered most likely a reflection of stress in the animals, as slight complement activation can occur from stress.
Table 2.
Complement analyses
| Sample | Bb, μg/ml | CH50, units/ml |
|---|---|---|
| Predose | 0.6 ± 0.2 | 242 ± 29 |
| D5W–5 min | 0.7 ± 0.2 | 216 ± 32 |
| D5W–1 h | 0.8 ± 0.3 | 252 ± 41 |
| Predose | 0.7 ± 0.0 | 270 ± 62 |
| 3 mg/kg–5 min | 0.7 ± 0.1 | 245 ± 45 |
| 3 mg/kg–1 h | 1.0 ± 0.2 | 256 ± 56 |
| Predose | 0.9 ± 0.2 | 196 ± 34 |
| 9 mg/kg–5 min | 0.9 ± 0.1 | 170 ± 45 |
| 9 mg/kg–1 h | 1.0 ± 0.0 | 241 ± 29 |
| Predose | 0.9 ± 0.1 | 254 ± 106 |
| 27 mg/kg–5 min | 1.0 ± 0.1 | 185 ± 95 |
| 27 mg/kg–1 h | 0.9 ± 0.1 | 247 ± 119 |
It is important to test for complement activation in non-human primates, which is one of the main reasons for investigating our targeted nanoparticles in this species. Oligonucleotides have shown species-specific responses between rodents and non-human primates (35), and antisense molecules encapsulated in cationic liposomes have revealed complement activation in cynomologous monkeys that was not observed in rabbits (36). The lack of complement activation observed from the targeted nanoparticles even at doses as high as 27 mg/kg suggests that complement activation will not be dose-limiting and correlates well with in vitro studies that revealed minimal complement activation (11).
Assessment of Immune Responses to the Targeted Nanoparticles.
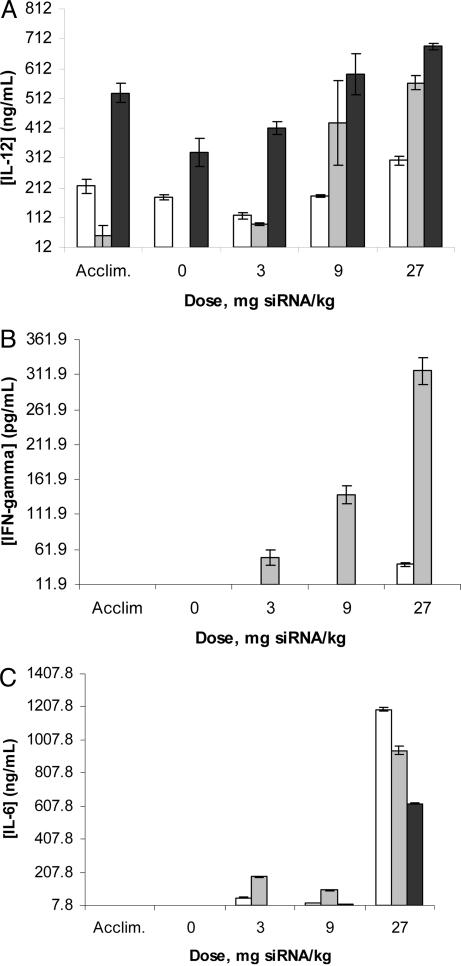
Cytokines were measured that can provide indications of T helper 1 (IL-12, IFN-γ), T helper 2 (IL-10, IL-4), and inflammatory responses (IL-6, TNF-α). IL-10, IL-4, and TNF-α levels were below the lower limits of quantification (1.8, 14.9, and 4.8 pg/ml, respectively) for all samples. The data for IL-12, IFN-γ and IL-6 are shown in Fig. 3. Elevated levels of IL-6 were observed in all three animals at the 27-mg/kg dose. Increasing amounts of IL-12 and IFN-γ were observed in one monkey at 9 and 27 mg/kg, respectively. However, the increases in these cytokines were not quantitatively impressive or suggestive of intensive immune stimulation. Taken together, these data suggest that targeted nanoparticles do not cause immune responses of significance at doses of 3 and 9 mg/kg (siRNA content), whereas modest elevation in specific cytokines was observed at the 27-mg/kg dose. Of importance is the fact that the siRNA used here is not chemically modified. We have shown that targeted delivery of siRNA that is not chemically modified can be accomplished in mice without immune stimulation, even when the siRNA contains a known immunostimulatory motif (19). In the present study, we observed a suggestion of limited immunostimulation in monkeys at a high dose level associated with other manifestations of toxicity, and under conditions of repeated (escalating) doses.
Fig. 3.
Cytokines. At 6 h postdosing, blood was drawn from each animal, processed into plasma, and analyzed for the indicated cytokine levels via ELISA (IL-6 and IL-12) or multiplex assay (IFN-γ). Experimentally determined lower limits of quantification values were 12 ng/ml for IL-12, 11.9 pg/ml for IFN-γ, and 7.8 ng/ml for IL-6. Bars indicate averages, and error bars indicate standard deviations for each group of n = 3 measurements (one for each of the three animals).
Multiple Doses of Targeted Nanoparticles Safely Administered in Non-Human Primates.
The hematological, serum, coagulation, complement activation, and cytokine parameter data taken together show that multiple doses of the targeted nanoparticles can be safely administered to non-human primates. Recently, work published by Grimm et al. (37) and Formstecher et al. (38) highlights the effects of saturating the RNAi pathways. Grimm et al. used expressed short hairpin RNAs in the liver of mice, whereas Formstecher et al. used synthetic siRNAs in vitro. Here, we do not observe physiological signs of toxicity that would suggest that the RRM2 siRNA is having negative effects on healthy tissue. Additionally, the safety of the nanoparticles at levels far above the amounts that were efficacious in mouse studies [0.5–1.0-mg/kg equivalents in monkey doses when extrapolated across species on a body surface area basis (19, 39)] suggests that there should be a large therapeutic index (ratio of the lethal dose of 50% of the study population divided by the effective dose of 50% of the study population) for the targeted nanoparticles, even when using multidosing schedules.
Zimmermann et al. (6) have reported single doses of nontargeted, lipid-formulated siRNAs in cynomolgus monkeys. At dose levels of 2.5 mg siRNA/kg, only elevated AST and ALT levels were observed. However, the values of AST and ALT at 48 h after dosing were remarkably high (1,555 ± 1,727 and 1,167 ± 1,157, respectively) and consistent with significant hepatocellular toxicity, which suggests that the 2.5 mg siRNA/kg dose of that formulation is near or above the maximum tolerated single dose in monkeys. No similar serum enzyme increases were observed at a 1.0 mg siRNA/kg dose in that study, which suggests that the dose–response for hepatotoxicity may be relatively steep. By comparison, the nanoparticle delivery system tested in the present study was not associated with any indications of organ dysfunction or significant toxicity at siRNA doses up to 9 mg/kg.
Antibody Formation and PK.
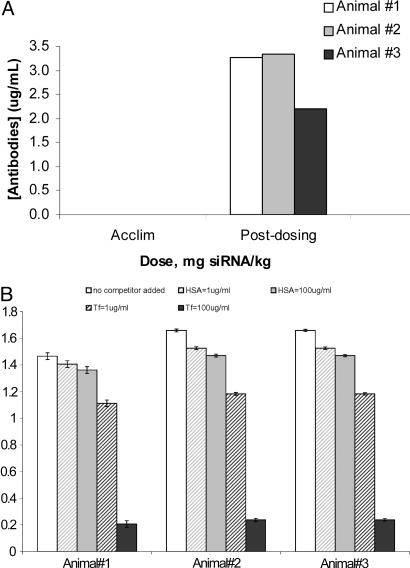
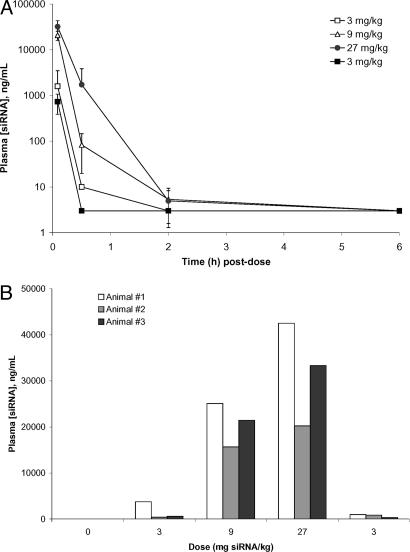
Fig. 4A shows data that reveal low titer antibody formation was obtained from all three animals. These antibodies react with the Tf on the nanoparticle, as their binding can be inhibited by the presence of Tf (Fig. 4B). Because antibody binding to the nanoparticle can be inhibited by Tf, it is suggestive that the antibodies are not binding to the PEGylated particle, but rather the human Tf. The antibody response is small and does not produce any manisfestations of a hypersensitivity reaction upon readministration of the targeted nanoparticles. Pharmacokinetic measurements provide an indication of the circulating half-life of the siRNA-containing nanoparticles and whether or not neutralizing antibodies cause accelerated blood clearance. The hybridization assay that was applied is specific for the antisense strand of the RRM2 siRNA (these assays are, by their nature, strand-specific, as sample processing causes strand separation of the RNA duplex). However, gel electrophoresis of selected monkey plasma samples revealed that the duplex siRNA remained encapsulated within the nanoparticles (data not shown). Thus, the hybridization assay yielded information on the plasma concentration of the siRNA that was associated with nanoparticles. The bioanalytical method includes a detergent treatment step that disrupts the nanoparticles and releases the siRNA. This method was used for analyzing monkey plasma samples taken predose and 5 min, 30 min, 2 h, and 6 h postdose (Fig. 5A). All three animals exhibit dose-dependent siRNA levels in plasma at the first (5 min) time point and relatively rapid clearance. Bellocq et al.¶ used this delivery system to deliver plasmids containing the human p53 gene into tumors of mice. In that study, PCR was used to determine the PK and biodistribution of the plasmid in whole blood after i.v. injection. The dose was equivalent to 2.5-mg/kg plasmid content in a monkey, scaled on a body surface area basis. It was found that, at 1 h postinjection, 1.4% of the injected dose was in the blood and that it declined to 0.01% at 24 h. That dose provided tumor delivery of plasmid DNA and detectable p53 mRNA (by RT-PCR) in the tumor at 24 h postinjection.¶ The work of Bellocq et al. is consistent with the data here with respect to the indications of rapid blood clearance of the nanoparticles. Very recently, Sato et al. (41) reported that galactose-containing liposomes (intended for directed uptake by the liver) are quickly eliminated from the blood of mice after i.v. injection. Those investigators measured the siRNA in whole blood, not plasma. Thus, targeted nanoparticle systems appear to be rapidly removed from the circulation, most likely owing to the intended tissue targeting.
Fig. 4.
Detection of monkey antibodies against human Tf. (A) Total antinanoparticle antibody titer was determined by ELISA on serum samples taken predosing (acclimation) and on day 18. (B) Serum samples (day 18) were incubated with human holo-Tf (1 or 100 μg/ml) or HSA (1 or 100 μg/ml) before antibody titer determination by ELISA. Incubation with HSA had a minimal effect on the ELISA signal, whereas there was a sharp dose-dependent reduction in the ELISA signal upon Tf incubation. This result supports the rationale that the antibodies generated specifically recognize the Tf moiety of the AD-PEG-Tf component of the nanoparticles.
Fig. 5.
PK of nanoparticles in monkey plasma. (A) Total plasma siRNA (ng/ml) was determined in monkey plasma as a function of time after dosing by hybridization-ligation assay. In all three animals, the t = 5 min plasma level scales with dose. Points represent the mean value for three animals, and error bars denote standard deviations. For all measurements below the lower limit of quantification (LLOQ), a value equal to half of the LLOQ (LLOQ/2) is reported. (Note: As discussed in Results and Discussion, there is reason to believe these values are underestimates of true circulating nanoparticle concentrations.) (B) Total plasma siRNA (ng/ml) concentrations at t = 5 min postdose. There is no clear difference observed between the initial (days 3 or 4) and final (day 21) 3-mg/ml dose across the three animals, suggesting that there may not be a reduction in half-life resulting from anti-Tf antibodies.
A comparison of the amounts of siRNA measured at 5 min after the initial and final 3-mg/kg doses (Fig. 5B) showed no significant differences between these dosing occasions, suggesting that there was no antibody-mediated acceleration of nanoparticle clearance. However, because of the limited number of animals and data points, a firm conclusion cannot be drawn regarding the possible influence of anti-Tf antibody formation on nanoparticle kinetics; further experiments that address this issue need to be performed. Also, it is important to note that no adverse reactions were observed in the animals from the final 3-mg/kg dose when antibodies were present. This result contrasts with the adverse reactions observed with repeated administration of lipid-based systems that are associated with the presence of antibodies (22, 23, 25–28).
Materials and Methods
Formulation of Targeted, siRNA-Containing Nanoparticles.
The targeted, nanoparticle formulation used here is the same as was used to show sequence-specific gene knockdown in mice at 2.5 mg/kg (19). The delivery system uses three components [cyclodextrin-containing polycation (CDP), adamatane (AD)-PEG, and AD-PEG-Tf; see Fig. 1] that are a physical mixture of 1:1 AD/β-CD (mol/mol) and AD-PEG/AD-PEG-Tf of 1:1,000 (wt/wt) (19). The mixture was added to an equal volume [all in 5% (wt/vol) glucose (D5W)] of siRNA (see Fig. 1) at a charge ratio (positive charges from CDP to negative charges from siRNA backbone) of 3:1 (±). Human holo-Tf was used as the targeting agent and is able to bind to mouse, rat, and monkey TfR (data provided in SI Fig. 6).
siRNA.
Synthetic siRNA (nonchemically modified) was purchased in gram quantities from Qiagen (Valencia, CA). The sequence used was: 5′-gauuuagccaagaaguucagu-3′ (sense) and 3′-cgcuaaaucgguucuucaagu-5′ (antisense).
This siRNA targets the RRM2 (34). This sequence has been shown to be a potent inhibitor of the RRM2 protein and exhibits significant antiproliferative activity in cancer cells of varying human types and species (mouse, rat, and monkey) (34). Antiproliferation data for human and monkey cancer cell lines are given in SI Fig. 7.
Treatment of Monkeys with Targeted, siRNA-Containing Nanoparticles.
All procedures involving cynomolgus monkeys were per-zoutf;Fn2formed by a contract research organization in accordance with applicable laws and regulations and under the guidance of the Institutional Animal Care and Use Committee. Animals received sequential single doses of nanoparticles at 0 (D5W only), 3, 9, and 27 mg/kg (with respect to siRNA content) according to the schedule depicted in Fig. 2; all formulations were prepared freshly on the day of injection at an siRNA concentration of 1 mg/ml. The 3- and 9-mg/kg injections were made via a standard “slow push” i.v. injection. The 27-mg/kg injection necessitated a dosing volume such that an i.v. infusion (2 h) was required; the 0-mg/kg (D5W only) dosing was done with the same volume and in the same fashion (2-h infusion) as the 27-mg/kg dosing. Each escalating dose was given after a 2- to 3-day washout period. After the 27-mg/kg dose was administered, an 11- to 12-day washout period was implemented to allow sufficient time for a possible antibody response to develop before one final 3-mg/kg dose.
Blood samples were taken at various time points relative to each of the doses and processed appropriately to allow numerous assessments, as described below. For hematology and coagulation, blood samples were taken once during acclimation (before the initial 0-mg/kg dosing) and 24 h postdose for the 0-, 3-, 9-, and 27-mg/kg doses; citrated plasma was obtained from whole blood for measurement of coagulation parameters. For serum chemistry, blood samples were taken once during acclimation and 24 h postdose for the 0-, 3-, 9-, and 27-mg/kg doses; serum was obtained from whole blood for measurement of serum chemistry parameters. For complement analyses, blood samples were taken predose and 5 min and 1 h postdose for the 0-, 3-, and 9-mg/kg doses and predose, immediately postinfusion, and 1 h postinfusion for the 27-mg/kg dose (given by 2-h infusion). The timing of the sampling for complement factors was based on the known propensity for activation to occur acutely in relation to oligonucleotide or excipient blood concentrations (i.e., when observed with i.v. injection of unformulated oligonucleotides or various other complement-activating formulations).
Analyses of Monkey Blood Samples.
Hematology and coagulation.
Hematological measurements were made on whole blood placed in an EDTA-treated tube and analyzed with an Advia 120 automated analyzer (Bayer Diagnostics, Terrytown, NY). Measured hematology parameters included erythrocyte count, hematocrit, hemoglobin concentration, leukocyte count (including five-part differential), mean platelet volume, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelet count, red cell distribution width, and reticulocyte count. Coagulation parameters evaluated in citrated plasma samples were assessed with a STACompacy automated analyzer (Roche Centralized Diagnostics, Basel, Switzerland); the measured coagulation parameters include activated partial thromboplastin time, prothrombin time, and fibrinogen.
Serum chemistry.
Serum chemistry determinations were made with an AU400 automated analyzer (Olympus, Melville, NY). The measured serum chemistry parameters included albumin/globulin ratio, albumin, ALT, alkaline phosphatase, AST, bilirubin (total), BUN, calcium, chloride, cholesterol (total), CRE, creatine kinase, gamma glutamyl transferase, globulin, glucose, phosphoruc (inorganic), potassium, protein (total), sodium, and triglyceride.
Complement factors.
Plasma Bb levels were determined by ELISA; serum CH50 values were determined by a standard sheep erythryocyte lysis-based assay.
Antibody analysis.
An ELISA was created in which 96-well plates were coated with Neutravidin and then treated with biotinylated nanoparticles. Nanoparticles were prepared as above using AD-PEG-Tf that had been biotinylated (1–5 biotins per AD-PEG-Tf) using a commercially available kit (Pierce, Rockford, IL). Animal serum samples were placed in nanoparticle-treated plates, followed by subsequent addition of HRP-conjugated protein A/G and the HRP substrate TMB. Absorbance measurements were made at 450 nm. A rabbit anti-human Tf anti-serum (Bethyl Laboratories) containing 1 mg/ml anti-human Tf polyclonal antibody was used as a positive control and to create a calibration curve. For competitive ELISA analyses, animal sera were incubated with either 1 or 100 μg/ml Tf or human serum albumin (HSA) before delivery to nanoparticle-treated plates.
Cytokines.
Serum IL-6 and IL-12 levels were determined by using commercially available ELISA kits (Invitrogen, Carlsbad, CA). Serum IFN-γ, IL-10, IL-4, and TNF-α levels were determined by using commercially available multiplex kits and a Luminex instrument (Invitrogen).
PK.
Plasma concentrations of the siRNA were determined by using a modified hybridization-ligation assay (40). The method was partially validated for accurate quantification of both naked and nanoparticle-contained siRNA from monkey plasma.
Supplementary Material
Acknowledgments
We thank Michael Kalos and Shu Mi of the City of Hope National Cancer Center (Duarte, CA) for cytokine measurements; Patricia Giclas and Cindy Marschner of the National Jewish Center (Denver, CO) for complement measurements; Sandra Carriero, Alvira Macanovic, and Helen Legakis of Charles River Laboratories Preclinical Services (Montreal, QC, Canada) for pharmacokinetic measurements; and Jodie Cowan and others at Shin Nippon Biomedical Laboratories USA (Everett, WA) for the in-life experiment and serum and hematology measurements.
Abbreviations
- Tf
transferrin
- TfR
Tf receptor
- RRM2
M2 subunit of ribonucleotide reductase
- CDP
cyclodextrin-containing polycation
- BUN
blood urea nitrogen
- CRE
creatinine
- ALT
alanine amino transferase
- AST
aspartate transaminase
- PK
pharmacokinetics
- AD
adamatane
- HSA
human serum albumin.
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 25, 2006.
Conflict of interest statement: M.E.D. is a consultant to and has stock in Calando Pharmaceuticals.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701458104/DC1.
Bellocq NC, Davis ME, Engler H, Jensen GS, Liu A, Machemer T, Maneval DC, Quijano E, Pun SH, Schluep T, Wen S (2003) Mol Ther 7:S290 (abstr).
References
- 1.Bumcrot D, Manoharan M, Koteliansky V, Sah DWY. Nat Chem Biol. 2006;2:711–719. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnaud CA. Chem Eng News. 2006;84:16–23. [Google Scholar]
- 3.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, et al. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 4.Morrissey DV, Blanchard K, Shaw L, Jensen K, Lockridge JA, Dickinson B, McSwiggen JA, Vargeese C, Bowman K, Shaffer CS, et al. Hepatology. 2005;41:1349–1356. doi: 10.1002/hep.20702. [DOI] [PubMed] [Google Scholar]
- 5.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, et al. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann TS, Lee ACH, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, et al. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda Y, Taira K. Pharm Res. 2006;23:1631–1640. doi: 10.1007/s11095-006-9001-x. [DOI] [PubMed] [Google Scholar]
- 8.Song E, Zhu P, Lee S-K, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, et al. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 9.O'McNamara JO, II, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Nat Biotechnol. 2006;24:1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 10.Chu TC, Twu KY, Ellington AD, Levy M. Nucleic Acids Res. 2006;34:e73. doi: 10.1093/nar/gkl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett DW, Davis ME. Bioconjugate Chem. 2007 doi: 10.1021/bc0603539. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Tang Q, Cheng D, Qin C, Xie FY, Wei Q, Xu J, Liu Y, Zheng B, Woodle MC, et al. Nat Med. 2005;11:944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidel JD, Hu S, Liu XF, Triche TJ, Davis ME. Nat Biotechnol. 2004;22:1579–1582. doi: 10.1038/nbt1038. [DOI] [PubMed] [Google Scholar]
- 14.Sioud M, Sorensen DR. Biochem Biophys Res Commun. 2003;312:1220–1225. doi: 10.1016/j.bbrc.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 15.Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 16.Sioud M. J Mol Biol. 2005;348:1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Robbins MA, Li M, Leung I, Li H, Boyer DV, Song Y, Behlke MA, Rossi JJ. Nat Biotechnol. 2006;24:566–571. doi: 10.1038/nbt1206. [DOI] [PubMed] [Google Scholar]
- 18.Judge AD, Bola G, Lee ACH, MacLachlan I. Mol Ther. 2006;13:494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ. Cancer Res. 2005;65:8984–8992. doi: 10.1158/0008-5472.CAN-05-0565. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni RP, Mishra S, Fraser SE, Davis ME. Bioconjugate Chem. 2005;16:986–994. doi: 10.1021/bc050081u. [DOI] [PubMed] [Google Scholar]
- 21.Mishra S, Heidel JD, Webster P, Davis ME. J Control Release. 2006;116:179–191. doi: 10.1016/j.jconrel.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Szebeni J, Baranyi L, Savay S, Bodo M, Morse DS, Basta M, Stahl GL, Bunger R, Alving CR. Am J Physiol. 2000;279:H1319–H1328. doi: 10.1152/ajpheart.2000.279.3.H1319. [DOI] [PubMed] [Google Scholar]
- 23.Szebeni J, Baranyi L, Savay S, Milosevits J, Bunger R, Laverman P, Metselaar JM, Storm G, Chanan-Khan A, Liebes L, et al. J Liposome Res. 2002;12:165–172. doi: 10.1081/lpr-120004790. [DOI] [PubMed] [Google Scholar]
- 24.Reasor MJ, Kacew S. Exp Biol Med. 2001;226:825–830. doi: 10.1177/153537020122600903. [DOI] [PubMed] [Google Scholar]
- 25.Semple SC, Harasym TO, Clow KA, Ansell SM, Klimuk SK, Hope MJ. J Pharmacol Exp Ther. 2005;312:1020–1026. doi: 10.1124/jpet.104.078113. [DOI] [PubMed] [Google Scholar]
- 26.Snoda K, Rydlewski J, Langner M, Kozubek A, Grzybek M, Sikorski AF. Cell Mol Biol Lett. 2005;10:37–47. [PubMed] [Google Scholar]
- 27.Ishida T, Ichihara M, Wang XY, Yamamoto K, Kimura J, Majima E, Kiwada H. J Control Release. 2006;112:15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Judge A, McClintock K, Phelps JR, MacLachlan I. Mol Ther. 2006;13:328–337. doi: 10.1016/j.ymthe.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Pun SH, Tack F, Bellocq NC, Cheng J, Grubbs BH, Jensen GS, Davis ME, Brewster M, Janicot M, Janssens B, et al. Cancer Biol Ther. 2004;3:641–650. doi: 10.4161/cbt.3.7.918. [DOI] [PubMed] [Google Scholar]
- 30.Gatter KC, Brown G, Trowbridge IS, Wollston R, Mason DY. J Clin Pathol. 1983;36:539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faulk WP, Taylor CG, Yeh CG, McIntyre JA. Mol Biother. 1990;2:57–60. [PubMed] [Google Scholar]
- 32.Head JF, Wang F, Elliott RL. Adv Enzyme Regul. 1997;37:147–169. doi: 10.1016/s0065-2571(96)00010-6. [DOI] [PubMed] [Google Scholar]
- 33.Rainov NG, Soling A. Curr Opin Mol Ther. 2005;7:483–492. [PubMed] [Google Scholar]
- 34.Heidel JD, Liu JYC, Yen Y, Zhou B, Heale BSE, Rossi JJ, Bartlett DW, Davis ME. Clin Cancer Res. 2007 doi: 10.1158/1078-0432.CCR-06-2218. in press. [DOI] [PubMed] [Google Scholar]
- 35.Henry SP, Giclas PC, Leeds J, Pangburn M, Auletta C, Levin AA, Kornbrust DJ. J Pharmacol Exp Ther. 1997;281:810–816. [PubMed] [Google Scholar]
- 36.Gokhale PC, Zhang C, Newsome JT, Pei J, Ahmad I, Rahman A, Dritschilo A, Kasid UN. Clin Cancer Res. 2002;8:3611–3621. [PubMed] [Google Scholar]
- 37.Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 38.Formstecher E, Reverdy C, Cholay M, Planquette C, Trouplin V, Lehrmann H, Arestea S, Calabrese A, Arar K, Daviet L, Colland F. Oligonucleotides. 2006;16:387–394. doi: 10.1089/oli.2006.16.387. [DOI] [PubMed] [Google Scholar]
- 39.Bartlett DW, Davis ME. Nucleic Acids Res. 2006;34:322–333. doi: 10.1093/nar/gkj439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu RZ, Baker B, Chappell A, Geary RS, Cheung E, Levin AA. Anal Biochem. 2002;304:19–25. doi: 10.1006/abio.2002.5576. [DOI] [PubMed] [Google Scholar]
- 41.Sato A, Takagi M, Shimamoto A, Kawakami S, Hashida M. Biomaterials. 2007;28:1434–1442. doi: 10.1016/j.biomaterials.2006.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.