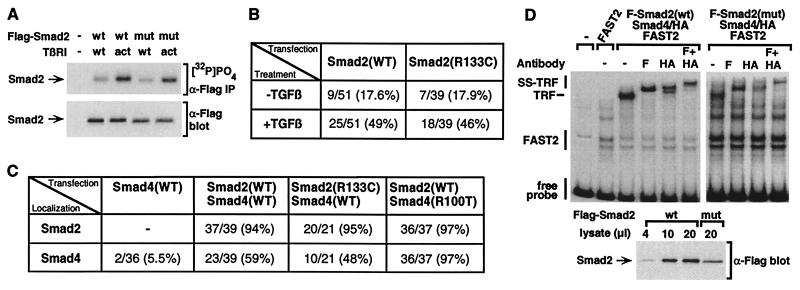
Figure 3.
Effect of the MH1 domain mutation on Smad2 phosphorylation, Smad nuclear translocation, and formation of a transcriptional activation complex. (A) Smad2(R133C) is phosphorylated by the TGF-β receptor. COS-1 cells transfected with wild-type (wt) or mutant (mut) Smad2 (1:2 DNA ratio) were labeled with [32P]PO4, and Smad2 was purified by immunoprecipitation with α-Flag antibody and analyzed by autoradiography ([32P]PO4, α-Flag IP). Total Smad2 protein levels were determined by immunoblotting of total cell lysates (α-Flag blot). (B and C) HepG2 cells were transfected with wild-type or mutant Flag-Smad2 (B) or with FAST2 together with wild-type or mutant Flag-Smad2 and Smad4-HA (C). Cells were incubated with (B and C) or without (B) TGF-β for 1 h, and the subcellular localization of expressed proteins was determined by immunofluorescence microscopy. The percentage of cells in which Smad2 (B and C) or Smad4 (C) displays an exclusively nuclear localization is shown. Because Smad2 recruits Smad4 into the nucleus, the decreased level of Smad4(R100T) protein allows the nuclear accumulation of the entire mutant Smad4 pool, thereby yielding an apparent increase in nuclear accumulation of Smad4(R100T) in comparison with the wild type. (D) EMSA. COS-1 cells were transfected with constitutively active TGF-β type I receptor, FAST2, Smad4, and either wild-type or mutant Smad (1:2 DNA ratio). Equivalent amounts of nuclear extracts were incubated with a 32P-labeled 121-bp gsc promoter probe, and protein/DNA complexes were separated by nondenaturing electrophoresis. For supershifting, α-Flag (F) or α-HA (HA) antibodies were added. Total Smad2 protein levels were determined by immunoblotting of aliquots of nuclear extracts (α-Flag blot).