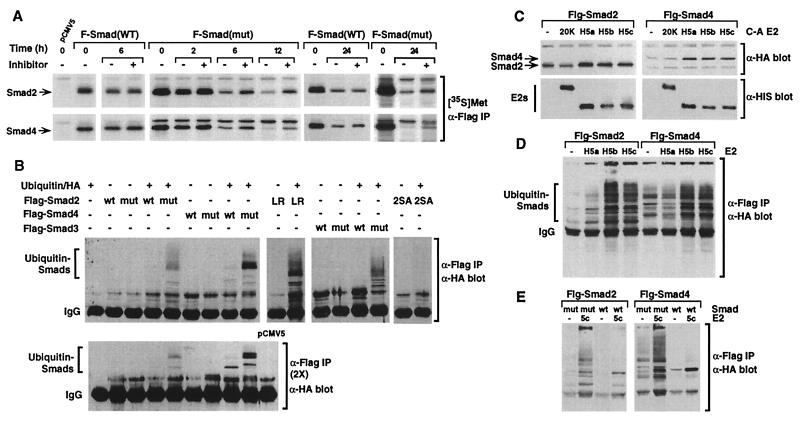
Figure 5.
The mutant Smads are degraded through the ubiquitin–proteasome pathway. (A) COS-1 cells transfected with Flag-tagged wild-type (WT) or mutant (mut) Smads were labeled with [35S]methionine for 30 min and then incubated in unlabeled culture medium with or without lactacystin for various times. Cell lysates were immunoprecipitated with α-Flag antibody, and [35S]methionine-labeled Smads were visualized by autoradiography ([35S]Met, α-Flag IP). (B) 293T cells transfected with Flag-tagged Smads and HA-tagged ubiquitin were immunoprecipitated with α-Flag antibody and ubiquitin-conjugated Smads were detected by immunoblotting with α-HA antibody (α-Flag IP, α-HA blot). Immunoprecipitates were boiled in SDS and reprecipitated with α-Flag antibodies before α-HA immunoblotting [α-Flag IP (2×), α-HA blot]. (C) COS-1 cells transfected with Flag-tagged Smads with or without the indicated E2 dominant-negative ligases (E2 C-A) were lysed, and steady-state Smad and E2 ligase protein levels were determined by immunoblotting with α-Flag or α-His antibodies. (D and E) 293T cells transfected with Flag-tagged Smads, HA-tagged ubiquitin, and wild-type E2s were immunoprecipitated with α-Flag antibody, and ubiquitin-conjugated Smads were detected by immunoblotting with α-HA antibody (α-Flag IP, α-HA blot).