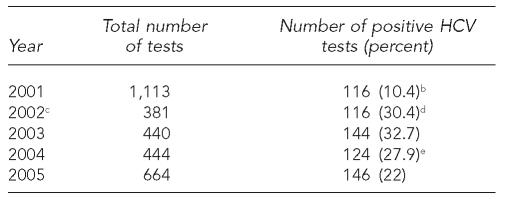
Table 4.
HCV testing and outcomes in STD clinics in Illinois,a by year, 2001–2005
Excluding Chicago.
An HCV test was defined as anti-HCV positive if it was reactive to both an enzyme immunoassay (EIA) and a recombinant immunoblot assay (RIBA).
In 2002, new screening criteria was introduced: only injecting drug users are eligible for HCV testing.
In July 2002, the Illinois Department of Public Health (IDPH) began using anti-HCV antibody signal-to-cutoff (S/CO) ratio to reduce testing costs. The new definitions of anti-HCV positive were (1) EIA reactive with a S/CO ratio >3.8 or (2) EIA reactive with a S/CO ratio ≤3.8 and RIBA reactive.
In 2004, IDPH began using an Ortho Vitros assay. The new definitions of anti-HCV positive were (1) EIA reactive with a S/CO ratio >8.0 or (2) EIA reactive with a S/CO ratio ≤8.0 and RIBA reactive.
HCV = hepatitis C virus
STD = sexually transmitted disease