SYNOPSIS
In 1999, the Florida State Legislature established and funded the statewide Hepatitis Prevention Program (HPP) to address growing concern about hepatitis C virus (HCV) and its potential public health burden. HPP supports county health departments' (CHDs') provision of viral hepatitis prevention services to at-risk adults through free hepatitis A and B vaccine in most CHDs and hepatitis serologic testing and statewide viral hepatitis-related education, consultation, and referral services. Some CHDs are directly funded by HPP.
In 2001–2005, HPP support helped CHDs provide 59,228 hepatitis A and 74,039 hepatitis B vaccinations statewide. In 2005, HPP supported almost 17,000 hepatitis B and C tests. From January to June 2005, 1,603 positive HCV tests were reported, a 9.5% seropositivity rate.
With $24 million from the Florida State Legislature through 2006, HPP has helped CHDs statewide provide substantial viral hepatitis prevention services to at-risk adults.
Because of concerns about hepatitis C virus (HCV) infection in Florida, in 1998, the Florida State Legislature and the Florida Department of Health (FDOH) considered possible hepatitis prevention programs. This consideration, along with the perceived need for a dedicated hepatitis prevention program and the availability of funds, resulted in an appropriation of $2.5 million by the Legislature to establish the Florida Hepatitis and Liver Failure Prevention and Control Program (Hepatitis Prevention Program [HPP] hereafter). HPP was designed to support county health departments' (CHDs') provision of viral hepatitis prevention services to at-risk adults1 through hepatitis A and B vaccine; hepatitis serologic testing; and statewide viral hepatitis-related education efforts, consultation, and referral services (e.g., drug treatment, social support, medical evaluation). HPP does not pay for medical evaluation or treatment. The Legislature has continued to support HPP by appropriating $3.5 million in fiscal years (FY) 2000 and 2001 and $3.4 million annually thereafter, for a total of $24 million through FY 2006.
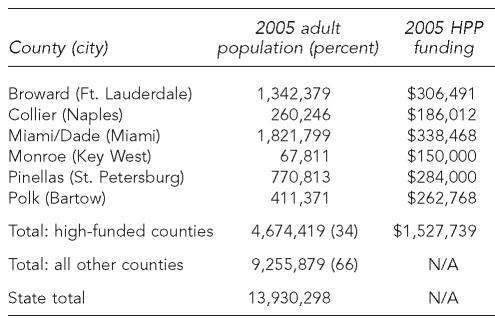
HPP directly funds 13 Florida CHDs to support hepatitis prevention activities in those counties. Since HPP started in 1999, six populous counties (Broward, Collier, Miami-Dade, Monroe, Pinellas, and Polk) have received substantial annual funding to support dedicated HPP staff—including a hepatitis program coordinator, nurse, and clerk—and to purchase hepatitis A and B vaccine and serologic testing for hepatitis A, B, and C. In 2005, the six high-funded counties received $150,000 to $338,000 in direct HPP funding for a total of $1.5 million, approximately half of the annual HPP appropriation (Table 1). The high-funded counties included about one-third of Florida's adult population aged 18 and older in 2005 (4.7 of 13.9 million).2
Table 1.
Florida counties, by 2005 adult population (aged 18 and older) and direct funding amount
HPP = Hepatitis Prevention Program
N/A = not applicable
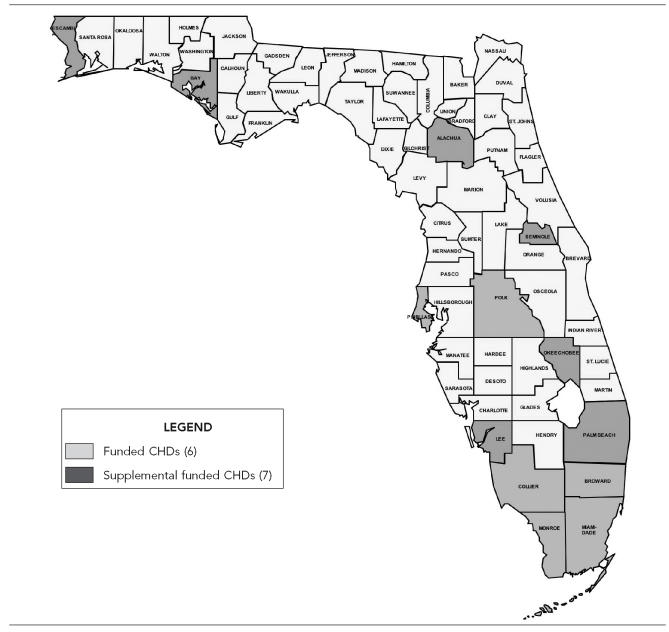
The seven other directly funded CHDs have received substantially less supplemental funding than the six high-funded CHDs, and for the purposes of this article, have been combined with the remaining 54 counties that do not receive direct HPP funding (hereafter “low-funded counties”). The low-funded counties receive free hepatitis A and B vaccine and hepatitis A, B, and C serologic testing from HPP and can use general revenue funds to support other hepatitis services. To receive these free HPP services, CHDs are required to submit a completed individual risk assessment for each vaccination or serologic test performed. A map showing Florida's 67 counties by HPP funding level is shown in the Figure.
Figure.
Florida Hepatitis Prevention Program, funded counties
PROGRAM ACTIVITIES
The Florida Viral Hepatitis Council, with 20 community, academic, and health department members, was established in 2004 to advise HPP.3 The Council developed a statewide strategic plan for 2005–2007 that sets specific goals and objectives to guide viral hepatitis prevention and control in Florida. The plan is updated every year.
Hepatitis A and B vaccination
HPP allocates adult hepatitis A and B vaccine to CHDs based on the counties' past use of vaccine as recorded in the Florida Health Clinic Management System, an integrated statewide clinic management system designed to provide service delivery and operational support to the CHDs through specific modules (e.g., billing, immunization, laboratory). The vaccine allocations are updated annually and are used to estimate vaccine orders for the state and prevent the accumulation of vaccine nearing expiration. Monthly, CHDs must account for every dose of vaccine they receive from HPP.
Many CHDs draw blood for serologic testing when they administer the first vaccine dose. Because the FDOH program purchasing policy is to procure hepatitis A and hepatitis B vaccine evenly from the two U.S. manufacturers, and because of cost considerations, almost all CHDs use monovalent hepatitis A or B vaccine rather than combined hepatitis A and B vaccine.
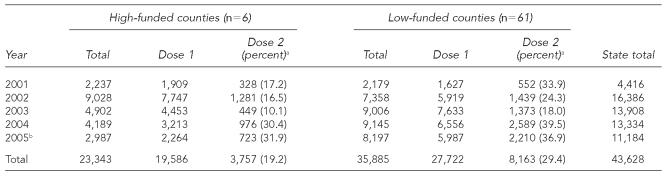
From 2001 to 2005, the statewide annual mean number of hepatitis A vaccinations was 8,726 (range: 4,416 to 16,386) (Table 2). Based on 2005 population data, the annual average rate of hepatitis A vaccination per 100,000 population was 99.9 in high-funded counties and 77.5 in low-funded counties, although the average estimated completion rate for the two-dose series was higher in low-funded counties (29.4% vs. 19.2%).
Table 2.
Number of monovalent hepatitis A vaccinations, by funding category and dose number, 2001–2005
Percentage is the ratio of the vaccine series, which is equal to the number of second doses divided by the number of first doses multiplied by 100.
Clinic services in many county health departments were impacted by four hurricanes in 2005.
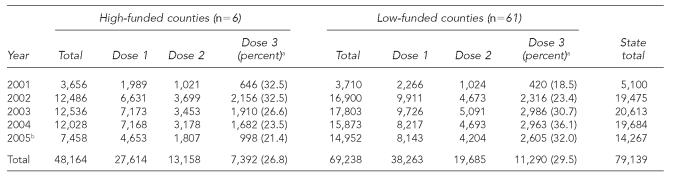
From 2001 to 2005, the statewide annual average number of hepatitis B vaccinations was 15,828 (range: 5,100 to 20,613) (Table 3). Based on 2005 population data, the annual average rate of hepatitis B vaccination per 100,000 population was about three times higher in high-funded counties (206.1, 48,164/4.67 million population) than in low-funded counties (66.9, 38,263/9.26 million), although the mean estimated completion rate for the three-dose series was similar (27% and 30%).
Table 3.
Number of monovalent hepatitis B vaccinations, by funding category and dose number, 2001–2005
Percentage is the ratio of third doses (i.e., rate of completion) relative to the number of first doses, which is equal to the number of third doses divided by the number of first doses multiplied by 100.
Clinic services in many county health departments were impacted by four hurricanes in 2005.
Hepatitis A, B, and C serologic testing
Beginning in March 2001, free hepatitis serologic testing through the FDOH state laboratory was offered to the 61 low-funded counties (the six high-funded counties were required to use HPP funds for testing). The initial testing panel included markers for hepatitis B virus (HBV) infection (hepatitis B core antibody [anti-HBc], hepatitis B surface antibody [anti-HBs], and hepatitis B surface antigen [HBsAg]) and HCV infection (antibodies to HCV, or anti-HCV). Beginning in late 2003, based on Centers for Disease Control and Prevention (CDC) guidelines for HCV testing and reporting, positive anti-HCV screening tests with a high signal-to-cutoff ratio were reported as positive without supplemental testing (e.g., recombinant immunoblot assay, polymerase chain reaction).4 In 2004, testing for hepatitis A virus (HAV) infection (anti-HAV) was added to the panel to allow serologic testing for immunity when the first dose of hepatitis A vaccine was given. Serologic data are maintained in a database that links data from client-completed risk assessments with laboratory testing results for the low-funded counties. Serologic testing data are not available for the six high-funded counties.
The 61 low-funded counties conducted 13,429 tests for anti-HAV in 2004 and 16,806 tests from January to June 2005. During this 18-month period, 7,873 of 30,235 (26%) anti-HAV tests were positive. There was a large increase in the number of anti-HBc tests conducted from 2002 (9,058), the first full year in which free hepatitis B testing was available to CHDs, to the first six months of 2005 (16,873); however, the proportion of positive anti-HBc tests varied little over this period (11.1% to 13.9%) (Table 4). The proportion of people testing positive for anti-HBc who also tested positive for HBsAg ranged from 7.6% to 10.0% (or 0.9% to 1.1% of all people tested for anti-HBc).
Table 4.
Hepatitis B testing and outcomes in low-funded counties, 2001–2005a
Hepatitis Prevention Program does not track serologic testing in the six high-funded counties.
Among those with positive core antibody tests.
Among those with positive core antibody tests who were tested for surface antigen.
Through June 30, 2005.
anti-HBc = hepatitis B core antibody
HBsAg = hepatitis B surface antigen
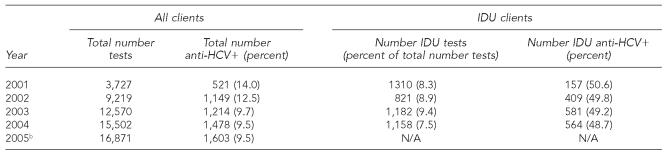
The total number of HCV tests in low-funded counties increased from 9,219 in 2002 to 16,871 in the first six months of 2005, but the proportion of positive tests decreased from 12.5% (1,149 positive tests) to 9.5% (1,603 positive tests) (Table 5). The percentage of all HCV tests that were among self-reported injection drug users (IDUs) ranged from 7.5% (2004) to 9.4% (2003), and the anti-HCV prevalence among IDUs tested was similar each year, for a mean of 49.3%. In contrast, the anti-HCV positivity rate among people who did not disclose injection drug use ranged from 5.5% (633/11,388 tests in 2003) to 10.7% (364/3,417 tests in 2001) during this time period (data not shown).
Table 5.
Hepatitis C testing and outcomes in low-funded counties, 2001–2005a
Hepatitis Prevention Program does not track serologic testing in the six high-funded counties.
Through June 30, 2005.
IDU = injection drug user
anti-HCV = antibody to hepatitis C virus
Referrals and follow-up of people with positive anti-HCV tests
Clients with positive anti-HCV tests were counseled on risk reduction, reduction of alcohol intake, available treatments, the need for medical evaluation, and the benefits of receiving hepatitis A and B vaccinations. Clients in very few counties were reported to have access to clinicians who provide medical evaluation on a sliding fee, and many clients with positive anti-HCV tests seen in CHDs were reported to have limited or no health insurance. Therefore, many of these clients are unlikely to be able to access medical evaluation or treatment. To help address this gap, in 2004, HPP helped establish the Florida Hepatitis Collaboration, Assessment, Resources and Education (HEP-CARE) project—a partnership between FDOH, the University of Florida, and pharmaceutical companies—to provide hepatitis care and treatment to indigent clients who are medically qualified. The pilot site for Florida HEP-CARE is Alachua County (Gainesville, Florida).
Since the program began in February 2005, 47 clients with positive anti-HCV tests have been referred for medical evaluation and possible treatment. Of these, 21 clients were started on treatment with long-acting interferon and ribavirin. As of September 2006, six clients have completed treatment. One achieved a sustained virologic response (undetectable virus six months after the end of treatment) and one relapsed. Four others had undetectable levels of virus at the end of treatment, and follow-up to determine whether they are sustained responders is underway. If this pilot is successful, Florida HEP-CARE may be expanded to other counties.
Training initiatives
HPP has sponsored two distance-learning opportunities to help ensure that CHD staff is trained to provide comprehensive viral hepatitis prevention services. Since November 2003, HPP has offered a quarterly Web-based training, “Hepatitis 101 for Counselors and Outreach Workers.” Each session trains 40 to 50 participants, and approximately 450 staff members have been trained to date. Since 2004, HPP has conducted eight sessions of a “Viral Hepatitis Serology Workshop” teleconference on serologic testing and interpretation of test results with a total of 225 participants, including CHD physicians, nurses, and epidemiologists, and clinical laboratory and emergency medical staff members.
Quality improvement
HPP provides assistance for some of FDOH's quality improvement (QI) site visits to help build local capacity for viral hepatitis integration. Large counties, including the six high-funded ones, usually receive annual QI site visits, and smaller counties may receive a site visit every two to three years. QI site visits are used to review program activities and identify technical assistance and training needs. For example, QI identified a client flow problem in a sexually transmitted disease (STD) clinic for clients who returned specifically for second or third doses of vaccine. These clients had to wait a long time to be seen, so a vaccine cooler was provided in the STD clinic so nurses could vaccinate on-site. In addition, HPP learned through QI site visits that, because resources for medical evaluation and treatment are nonexistent, many CHDs are concerned about HCV testing.
DISCUSSION
HPP was created in 1999 to address growing concern about viral hepatitis and its potential public health burden in Florida. Funded and managed at the state level, HPP has been able to expand services largely because of its close collaboration with the CHDs, whose staff are state employees, and various state entities, including the Bureaus of HIV/AIDS (within which HPP is now located), Epidemiology, Immunization, and the Tuberculosis and Refugee Health programs. Since 1999, Florida has been one of three states that have received substantial legislative appropriations to support viral hepatitis prevention services. California had a one-time appropriation of $1.5 million for hepatitis C activities, which was expended by the end of 2003; in 1999, approximately $6 million was appropriated in Texas for hepatitis C education and services for 2000 to 2005.5,6 Only the Florida State Legislature has continued appropriations into 2006.
Viral hepatitis prevention services are offered in most of Florida's 67 CHDs through programs serving at-risk adults (e.g., human immunodeficiency virus [HIV], STD, immunization) and other programs serving a mixture of low- and at-risk adults (e.g., Family Health Services). Direct HPP funding for personnel was associated with greater hepatitis B vaccination in high-funded counties compared with other counties, although completion rates were comparable. In the 61 low-funded counties, partial data from 2005 show that, by year's end, the numbers of hepatitis A and B tests performed will be approximately twice the number performed in 2004. Florida is fortunate in that a low-cost hepatitis serology panel is available through the state public health laboratory, making it feasible to conduct widespread testing in CHDs. In other settings, however, it may not be economically feasible to conduct pre-vaccination testing for HAV and HBV. CDC recommends that the decision to conduct pre-vaccination testing be based on cost-effectiveness or a decision to identify individuals with chronic HBV infection.7
The decreasing anti-HCV positivity suggests that (1) increasing numbers of lower-risk people are being tested and/or (2) anti-HCV positivity among IDUs could be decreasing. If funds are limited and to minimize false positive results, CDC recommends targeting high-risk groups for HCV testing.8 However, because Florida has access to a low-cost testing panel that includes anti-HCV, the additional cost of anti-HCV testing is negligible. In addition, given that 5.5% to 10.7% of people tested who do not disclose injection drug use have positive anti-HCV results, CHD staff believe that continuing testing of some clients who do not disclose injecting drugs is valuable because some clients who do inject drugs do not want to disclose this risk factor.
There were three limitations to this assessment of viral hepatitis service delivery in Florida. First, serologic testing data were unavailable for the six high-funded counties and were not linked with vaccination data in the low-funded counties; therefore, it is unknown how many people who started the hepatitis A or B vaccine series discontinued it because they were later found to be immune. Second, data on the number of client visits to venues serving at-risk adults (e.g., STD clinics) or the number of at-risk client visits to more general public clinics (e.g., Family Health Services) were unavailable; thus, 2005 population data were used to calculate vaccination rates, which may not be representative of the population seeking services at CHDs. Third, because vaccine doses were not tracked for individuals, completion rates of each series are estimated based on numbers of first, second, and (for hepatitis B vaccine) third doses reported each calendar year.
Although the common barriers to viral hepatitis integration in existing public health programs—inability of health-care providers to deliver vaccine, lack of public health infrastructure, and insufficient provider knowledge—have largely been overcome, challenges remain. First, the absence of state or federal programs for medical evaluation and treatment for people living with chronic HCV infection means that many of the anti-HCV-positive adults identified in these public health settings were unlikely to obtain these services. Expansion of Florida HEP-CARE may help to address this gap. Second, although QI visits have helped improve HPP services in directly funded counties and other larger CHDs, lack of funding has meant that many CHDs have infrequent or no QI visits. Third, rural counties that do not have funded STD, HIV/acquired immunodeficiency syndrome (AIDS), or other integrated staff may benefit from viral hepatitis prevention services training. HPP has developed training on viral hepatitis and interpretation of serologic test results, and CHDs and outreach venues are encouraged to participate. Another challenge identified through QI site visits was that some CHD staff view providing hepatitis prevention services as an additional burden to the other services they are providing because of the extra time needed to explain viral hepatitis to clients and to complete the risk assessment. This is particularly true in small CHDs where there are limited staffing resources.
Florida's HPP provides a good model of an almost entirely state-funded program that has been able to provide substantial viral hepatitis prevention services in clinical public health settings statewide. Principally provided in STD, HIV, and immunization settings, statewide services include hepatitis vaccination and serologic testing for at-risk adults. The Florida HPP illustrates how state resources can be mobilized to provide viral hepatitis services to at-risk adults.
REFERENCES
- 1.Atkinson W, Hamborsky J, McIntyre L, Wolfe S, editors. 9th ed. Washington (DC): Public Health Foundation; 2006. CDC epidemiology and prevention of vaccine-preventable diseases. [Google Scholar]
- 2.Florida Department of Health, Office of Planning, Evaluation – Data Analysis. [cited 2006 Sep 1];Community Health Assessment Resource Tool Set (CHARTS) Available from: URL: http://www.floridacharts.com/charts/chart.aspx.
- 3.Florida Department of Health. [cited 2006 Sep 1];The Florida Viral Hepatitis Council home page. Available from: URL: http://www.doh.state.fl.us/Disease_ctrl/aids/hep/VHCouncil/VHCouncil.html.
- 4.Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. MMWR Recomm Rep. 2003;52(RR-03):1–16. [PubMed] [Google Scholar]
- 5.Bill number: 1256 (Cal. 2000) [cited 2006 Dec 1]; Available from: URL: http://www.leginfo.ca.gov/pub/99-00/bill/sen/sb_1251-1300/sb_1256_bill_20000927_chaptered.html.
- 6.Heseltine G, McFarlane J. Texas statewide hepatitis C counseling and testing, 2000–2005. Public Health Rep. 2007;122(Suppl 2):6–11. doi: 10.1177/00333549071220S202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (US) A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP), part II: immunization of adults. [cited 2006 Dec 1];MMWR Recomm Rep. 2006 55(RR-16):1–25. Also available from: URL: http://www.cdc.gov/mmwr/PDF/rr/rr5516.pdf. [PubMed]
- 8.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. [cited 2006 Sep 1];MMWR Recomm Rep. 1998 47(RR-19):1–39. Also available from: URL: http://www.cdc.gov/mmwr/PDF/rr/rr4719.pdf. [PubMed]