Abstract
Forming new knowledge based on knowledge established through prior learning is a central feature of higher cognition that is captured in research on stimulus equivalence (SE). Numerous SE investigations show that reinforcing behavior under control of distinct sets of arbitrary conditional relations gives rise to stimulus control by new, derived relations. This investigation examined whether frontal-subcortical and frontal-parietal networks known to support reinforced conditional relations also support derived conditional relations. Twelve adult subjects completed matching-to-sample (MTS) training with correct/wrong feedback to establish four trained conditional relations within two distinct, three-member stimulus classes: (1) A1→B1, B1→C1 and (2) A2→B2, B2→C2. Afterwards, functional neuroimaging was performed when MTS trials were presented involving matching two identical circles (a sensorimotor control condition), trained relations (A→B, B→C), and derived relations: symmetry (B→A, C→B), transitivity (A→C), and equivalence (C→A). Conditional responding to trained and derived relations was similarly correlated with bilateral activation in the targeted networks. Comparing trained to derived relations, however, highlighted greater activation in several prefrontal regions, the caudate, thalamus, and putamen, which may represent the effects of extended training or feedback present during imaging. Each derived relation also evidenced a unique activation pattern. Collectively, the findings extend the role of frontal–subcortical and frontal–parietal networks to derived conditional relations and suggest that regional involvement varies with the type of derived conditional relation.
Keywords: stimulus equivalence, relational memory, conditional learning, visuomotor, neuroimaging, event-related fMRI, humans
Matching-to-sample (MTS) paradigms are commonly employed to investigate learning arbitrary conditional visual-visual relations. Training using a simultaneous MTS procedure, for example, involves presenting a sample stimulus (e.g., “A1”) alongside two or more comparison stimuli (e.g., “B1” and “B2”). To establish the arbitrary conditional stimulus relation A1→B1, choice of B1 in the presence of A1 produces reinforcement, whereas choice of B2 does not.
Basic and applied behavioral research on stimulus equivalence (SE) builds on conditional learning research in an important and innovative way. Baseline training employs reinforcement to establish several conditional or trained relations within separate stimulus classes, such as the following: Class 1 = A1→B1 and B1→C1; Class 2 = A2→B2 and B2→C2. Once a set of trained relations is established, stimuli are recombined and conditional responding is tested in a MTS format (e.g., with a C1 sample, A1 and A2 appear as comparisons). It has been consistently shown that a set of derived class-specific equivalence relations form among recombined stimuli as an outcome of the reinforcement contingencies (Sidman, 2000; but see Tonneau, Arreola, & Martinez, 2006, regarding the necessity of reinforcement in this conception of SE). These derived relations are commonly known as symmetry (e.g., B1→A1; C1→B1), transitivity (e.g., A1→C1), and equivalence (e.g., C1→A1) (alternatively, Hayes, Barnes-Holmes, & Roche, 2001, emphasize relational frames). Thus, the SE methodology provides an important window into the process of acquiring new knowledge derived from learned facts.
Many prominent behavior theories of psychopathology, concept formation, language, and human development are based on SE (Devany, Hayes, & Nelson, 1986; Friman, Hayes, & Wilson, 1998; Hayes, Barnes-Holmes, & Roche, 2001; Moerk, 1997; Plaud et al., 1998; Saunders, Drake, & Spradlin, 1999). Perhaps most importantly, the SE framework has provided clinicians a robust set of tools for developing a wide variety of deficit-specific clinical/educational interventions for individuals with cognitive dysfunction (Cowley, Green, & Braunling-McMorrow, 1992; Melchiori, de Souza, & de Rose, 2000; Saunders, O'Donnell, Vaidya, & Williams, 2003). However, although much is known about SE behaviorally, our understanding of the supporting neurobiology is limited. Clarifying the functional–anatomical substrates of SE promises to contribute to neurophysiological research on conditional learning and higher cognitive functioning, as well as expand our understanding of equivalence-based clinical treatments.
Numerous human and nonhuman neurophysiological studies on learning, memory (working, declarative, spatial, relational, recognition), and transitive inference suggest conditional responding to trained and derived relations recruits a set of frontal–parietal and frontal–subcortical networks central to higher cognition (Acuna, Eliassen, Donoghue, & Sanes, 2002; Brasted & Wise, 2004; Cabeza & Nyberg, 2000; Elliott & Dolan, 1999; Grossman et al., 2002; Inase, Li, Takashima, & Iijima, 2001; Lepage, Brodeur, & Bourgouin, 2003; McDermott, Jones, Peterson, Lageman, & Roediger, 2000; Murray, Gaffen, & Mishkin, 1993; Opitz & Friederici, 2003; Porter, Koch, & Mair, 2001; Ricci et al., 1999; Schlund, Pace, & McGready, 2001; Schlund & Pace, 2000; Toni, Ramnani, Josephs, Ashburner, & Passingham, 2001). Several anatomically distinct frontal–subcortical circuits exist, but the dorsolateral and ventrolateral frontal circuits are widely recognized as central to planning and execution of motor acts and nonmotor cognitive processes, such as attention, memory, and “executive” functions. These circuits project to the striatum (caudate and putamen), from there through the globus pallidus to specific thalamic nuclei, with a final link back to the frontal lobe. Prefrontal regions also have dense projections to premotor regions to facilitate and direct behavior. Within the prefrontal cortex, ventral portions receive vision-correlated input from the inferotemporal cortex, the neurons of which respond differentially based on the shape or color of visual stimuli. Importantly, disconnection from ventral prefrontal regions severely impairs acquisition and retention of arbitrary visual–motor, auditory–motor, and visual–visual relations (Bussey, Wise, & Murray, 2002; Eacott & Gaffan, 1992; Gaffan & Harrison, 1991). In contrast, dorsal portions receive visual input from the inferior parietal cortex in the dorsal visual stream, enabling accurate hand and eye movements, as well as nonmotor behaviors, such as directing attention covertly without an overt response. Dorsal lesions also do not impair visual matching (Mishkin & Manning, 1978; Passingham, 1975). Based on these functional-anatomical data, it is thought that the prefrontal cortex lies positioned at the top of the sensory and motor hierarchy for integrating stimuli, responses, and outcomes (Passingham, Toni, & Rushworth, 2000), with ventral prefrontal cortex activity potentially reflecting incoming sensory information and dorsal prefrontal cortex activity reflecting operant behavior. Prefrontal activity and responding to conditionally related stimuli is also then modified (strengthened or weakened) based upon striatal activity to consequences.
In this investigation, our primary aim was to employ blood-oxygen-level dependent functional magnetic resonance imaging (BOLD fMRI) to examine whether conditional responding to trained and derived relations recruits frontal–subcortical and frontal–parietal networks during conventional SE testing. Results of a recent fMRI study conducted by Dickins et al. (2001) are relevant in this regard. BOLD fMRI was employed to examine whether brain activation correlated with presentation of derived relations in a MTS format also recruited brain regions implicated in language. During baseline training, trained relations were established using a multistage errorless training protocol in which stimuli were correctly paired together, and then over subsequent trial blocks additional distractor stimuli and feedback were introduced. Functional neuroimaging occurred while subjects completed MTS trials involving a sensorimotor control relation that required matching two asterisks, and trained, symmetry, transitive, and equivalence relations. During separate imaging runs, 15-s blocks of control trials alternated with 15-s blocks of one other type of relation (e.g., symmetry). Separate imaging analyses contrasted activation correlated with blocks of each derived relation with activation correlated with blocks of the control relation to isolate brain regions showing significantly sustained increases in BOLD fMRI signal (e.g., symmetry > control). Results most relevant to our initial question were those showing activation in inferior frontal (dorsolateral) and inferior parietal regions for both trained and derived relations. Moreover, in subcortical regions, caudate activation was observed across trained and derived relations and activation was noted in the thalamus for trained and equivalence relations. Analyses directly comparing trained and derived relations were not performed, however, leaving open questions regarding differences in the extent and magnitude of activation between trained and derived relations in our regions of interest.
The investigation of Dickins et al. (2001) is encouraging, but several procedural and analysis issues limit interpretations of results. Of most concern is that the results were based on pooling imaging data across subjects. Comparisons or contrasts between conditions that revealed significant differences in activation were based on variability between scans rather than variability between subjects–thus, ‘subjects’ was not treated as a random factor in the analysis, which is the conventional approach used in neuroimaging analyses. As a result, Dickins et al.'s inferences apply only to the sample studied rather than the population from which the sample was drawn. A second concern is that the results were based on analyses of 15-s blocks of time that contained multiple MTS trials. Such block analyses are statistically powerful and not uncommon in neuroimaging research, but results reflect sustained BOLD signal during the 15-s period, which misses phasic event-correlated activity for particular critical events, such as choice of the correct comparison. The activation Dickins et al. reported reflects varying numbers of presentations of sample and comparison stimuli, visual search among comparisons, choice responding, intertrial intervals, and incomplete trials. Despite these limitations, the approach of Dickins et al. serves as a good reference point for further attempts to integrate SE MTS procedures with BOLD fMRI. To build on this effort, the second aim of this investigation was to employ conventional random-effects fMRI analyses that preserve individual subject effects. Block analyses of imaging data were omitted in favor of event-related analyses in which BOLD signal changes are correlated with the discrimination, and other, unrelated MTS events are ignored. Lastly, we examined the correspondence between group analyses and individual subject effects.
Method
Subjects
Twelve adult humans consented to participate (5 males and 7 females). All reported being between 18 and 50 years of age, right-handed, free of medications affecting the central or autonomic nervous system, and without a personal history of drug abuse, psychiatric disorder, or a psychiatric history in first-degree relatives. Eligible subjects were paid $10.00 per hr.
Apparatus
Training occurred on a color desktop computer with a MTS task programmed with Eprime software. Stimuli were black ASCII characters approximately 2.5 in high and wide. Subjects were trained individually in a small quiet room for a 2-hr session.
Procedure
The procedure included two steps: MTS baseline training and equivalence testing during neuroimaging. The following instructions were used during training and neuroimaging. Instructions were printed on the computer screen at the start of each session and initially read aloud by the experimenter:
This task is designed to help you learn ‘relations’ between different symbols. It will last [5–12] minutes and consists of many trials. During a trial, you will see a symbol printed on the left side of the screen. Moments later, you will see two more symbols appear on the right side of the screen (one located on the top and one on the bottom of the screen). When these symbols appear, you will have 4 s to make a choice. To make a choice: Press the top button (#1) if you believe the symbol on the left “goes with” the top symbol OR Press the bottom button(#2) if you believe the symbol on the left “goes with” the bottom symbol. After your choice, the computer will SOMETIMES print “Correct” or “Wrong.” Another trial will then be presented. So, the goal is to learn whether the symbol on the left “goes with” or is “related” to the top symbol or the bottom symbol. Please note: On some trials the position of symbols will change and you will see a different arrangement of symbols. Just pay close attention to all the symbols that appear and you will do very well. Lastly, on some trials you will see a black circle on the left side of the screen and only one black circle on the right side of the screen. In this case there is only one choice to make, so you should simply select the black circle that appears. Be sure to remember what symbols go together!! Later you will complete a memory test. Ready to start?
MTS Baseline Training
Task
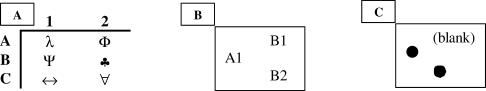
An MTS task with one sample stimulus and two comparison stimuli was employed during training and imaging. Figure 1A shows the six MTS stimuli used, and Figure 1B shows an MTS computer screen display. For clarity, we will refer to stimuli and stimulus relations using the letter–number combinations appearing in Figure 1A. Training was designed to establish four trained relations within two, three-member stimulus classes (A1→B1, B1→C1 and A2→B2, B2→C2). Each MTS trial consisted of presenting a sample stimulus followed 500 ms later by two comparison stimuli positioned vertically. The top–bottom position of comparison stimuli varied randomly across trials. Responding involved pressing button 1 on a keypad to select the top comparison and pressing button 2 on the keypad to select the bottom comparison. Responses were made using only the right hand. Throughout the investigation, the correct comparison stimulus was class-specific (e.g., given sample A1 choose comparison B1, not B2; B1 choose comparison C1, not C2), thus, the incorrect comparison stimulus was the correct comparison's corresponding cross-class equivalent. After MTS responding, the prompt “Correct” or “Wrong” appeared for 1 s, followed by a blank screen until the next trial.
Fig 1. (A) Six examples of MTS stimuli used during training and neuroimaging.
Each stimulus is designated by a letter–number combination. (B) Computer screen display for a MTS trial used during training and neuroimaging. (C) Screen display for a match relation control trial in which the sample and comparison were identical—match trials served as the sensorimotor control or baseline condition to evaluate increases in BOLD signal activation during discriminations of trained and derived relations.
Trials
Throughout the investigation, MTS trial durations also were varied between 5 s and 7 s (averaging 6 s). This variable trial duration, referred to as “jittering” the intertrial interval, is a conventional imaging design approach used to ensure that the fixed image acquisition rate begins at different points within MTS trials. Jitter eliminates sampling activation at the same time point on all MTS trials resulting in improved sampling of the hemodynamic response (e.g., Miezin, Maccotta, Ollinger, Petersen, & Buckner, 2000)
MATCH control trials
Instructions were used to establish a matching response between a black sample circle and a single identical black comparison circle. Figure 1C shows a computer screen display for a MATCH control trial. MATCH trials served as a sensorimotor control relation, which does not involve a conditional discrimination, for evaluating increases in activation correlated with trained and derived relations, which involve conditional discriminations.
Phases
MTS baseline training consisted of three phases. Within each phase, sessions consisted of 25 trials. In phase 1, each session was divided into consecutive blocks of six A1→B1 trials, six A2→B2 trials, and eight intermixed trial types (Mixed AB). In addition, five MATCH control trials were interspersed randomly within the session. Training ended when percent correct matching reached 100% for the Mixed block and a minimum of two or a maximum of three sessions were completed. Phase 2 was identical to phase 1 but involved B1→C1, B2→C2, and Mixed BC trials. Due to a procedural error, the stability criteria were violated for subject 9 during phase 1 training and subject 10 during phase 2 training. However, matching during the Mixed block was 100% accurate in both cases. In phase 3, each session contained ten Mixed AB trials, ten Mixed BC trials, and five MATCH trials presented in a randomized order. Training continued until the overall percent correct matching exceeded 85% for two consecutive sessions and 100% correct matching occurred for both AB and BC trial types in the final session. Data in The Appendix show the order of baseline training sessions and percent correct matching per session for each subject.
Functional Neuroimaging During Equivalence Testing
Task
An event-related fMRI design was employed. During imaging, subjects completed an identical version of the MTS task used in the final phase of training. Twenty of each of the following trial types were presented: matching control trials (matching two circles), training trials with correct/wrong feedback (A1→B1, B1→C1, A2→B2, B2→C2), symmetry trials (B1→A1, C1→B1, B2→A2, C2→B2), transitivity trials (A1→C1, A2→C2), and equivalence trials (C1→A1, C2→A2). Instructions were identical to those used during baseline training.
Apparatus and Parameters
Functional MRI images were obtained on a 3.0 T Philips MRI scanner. The MTS task was programmed with Eprime software. Instructions and stimuli were presented on a rear screen monitor viewed through a mirror anchored to a standard head coil. Subjects held a small box with two response buttons arranged vertically in their left hand and pressed response buttons with their right thumb. T1 anatomical volume images were first collected for each subject using a MPRAGE sequence with a high-resolution isovoxel acquisition of 1.0 mm3. Functional MRI data were gathered using a single shot echo planar imaging (EPI) sequence for data acquisition. Parameters were a repetition time (TR) of 3 s, an estimation time (TE) of 30 ms, a 90 degree flip angle, 128 × 128 matrix size and field of view 24 cm, yielding voxels measuring 3 × 3 mm in plane. Using these parameters, 39 contiguous 3 mm thick sections were obtained angled parallel to the intercommissural line. The first three volumes were discarded to allow for equilibration effects.
Imaging analysis
Two criteria must have been met for a subject's imaging data to be included in the analysis: (a) head displacement in x, y, and z planes must not have exceeded 2 mm during each scan (assessed using visual inspection of realignment parameters), and (b) the percentage of correct responding for a relation must have exceeded 75%. Data from Subjects 1 and 4 were excluded from primary analyses because they failed to reach the 75% correct responding criterion. Functional EPI images were first reconstructed from k-space to image space for further processing. All preprocessing and data analysis were performed using statistical parametric mapping software, version 2 (SPM2; Wellcome Department of Cognitive Neurology, London, UK). EPI images were slice-timing corrected to adjust for the lag between slices during each TR, corrected for head motion during scanning, and normalized to a standard template brain from the Montreal Neurological Institute (MNI) to get all participants into the same space (Friston et al., 1995). After coregistration and normalization, voxels were resampled with a 2 × 2 × 2 mm voxel size. EPI images then were spatially smoothed using a 6 mm full-width-half-maximum (FWHM) Gaussian kernel. High pass filtering (1/120 HZ) also was applied to the time series of EPI images to remove the low frequency drift in EPI signal.
Hypothesis-driven regional analyses
The focus of imaging analyses was to identify voxels that showed significantly greater activation (>) in one condition relative to a second condition. These are referred to as ‘contrasts’ and were as follows: (a) trained > MATCH, (b) symmetry > MATCH, (c) transitive > MATCH, (d) equivalence > MATCH, (e) derived > MATCH (with derived consisting of pooling data from symmetry, transitivity, and equivalence relations), (f) trained > derived, and (g) derived > trained.
To quantify the magnitude and extent of activation, a conventional two-level analysis was employed. At the first level, individual-subject models were constructed in which a linear regression analysis was performed between the observed event-related EPI signals and onset times of comparison stimuli and responding (Friston, Worsley, & Frackowiak, 1995). This produced a beta weight (regression coefficient) for each voxel. Beta weights were based on aggregated fMRI signal changes and approximate percent change in fMRI signal relative to a global fixed constant, which is an average value of all time points in a session. Using beta weights from the model, individual subject beta differences were calculated at each voxel for each contrast (e.g., for contrast (a) above: Beta Difference = Trained Beta – MATCH Beta). At the second level, voxel beta differences within each contrast (a–g) were grouped across subjects and a group mean beta difference calculated. Multiple one-sample t-tests were then employed to identify those voxels with mean differences significantly greater then zero (for an expanded discussion on analyses of fMRI data see Schlund & Cataldo, 2005; see also Demonet, Thierry, & Cardebat, 2005). Voxels considered to show activation were those exceeding the thresholds p < .005, uncorrected for multiple comparisons, and an extent threshold (k) of 15 contiguous voxels (Holmes & Friston, 1998). This analysis excluded all nonsignificant voxels and voxel clusters containing less than 15 contiguous voxels, effectively yielding an uncorrected cluster level threshold of p < .06. To further isolate event-related activity correlated with discriminations of trained and SE relations in frontal and parietal regions, a second set of more stringent one-sample t tests were conducted using statistical thresholds of p < .0005 (uncorrected) and an extent (k) of 15 contiguous voxels, which yields uncorrected cluster level threshold of p < .003. To interrogate subcortical regions interconnected to prefrontal regions, statistical thresholds of p < .005 (uncorrected) and an extent (k) of 40 contiguous voxels, which yields uncorrected cluster level threshold of p < .004, were employed.
Based on results of previous investigations, our a priori regions of interest were the inferior frontal gyrus and inferior parietal lobule complex, as well as subcortical regions including the thalamus, lentiform nucleus (globus pallidus and putamen), and caudate. Second-level analyses were restricted to regions of interest using anatomically defined masks created by the Wake Forest University PickAtlas (Maldjian, Laurienti, Burdette, & Kraft, 2003). This software employs SPM2's small volume correct feature to adjust results in accordance with the size of the region interrogated, thereby reducing the number of multiple comparisons performed. Finally, the locations of voxels with significant activation were summarized by their local maxima separated by at least 8 mm, and by converting the maxima coordinates from MNI to Talairach coordinate space using the formulas provided by Brett (2002). Finally, these coordinates were assigned neuroanatomic labels using the Talairach Daemon (Fox & Uecker, 2005) and human brain atlases.
Results
Behavioral
Tabled data in The Appendix provides detailed information on training sequences and percentages of correct responses for each subject. All trained relations (A1→B1, B1→C1, A2→B2, B2→C2) were established successfully for each subject prior to imaging. Behavioral results from SE testing during imaging appear in Table 1 and show 10/12 subjects consistently responded above 75% correct for each trial type. Subjects 1 and 4 failed equivalence testing and as a consequence, their data were excluded from group imaging analyses.
Table 1. Percent correct responses for Subjects 1–12 during neuroimaging.
| Subject | Match | Trained | Symmetry | Transitivity | Equivalence |
| 1* | 100 | 91 | 75 | 4 | 0 |
| 2 | 100 | 100 | 100 | 100 | 100 |
| 3 | 100 | 100 | 87 | 88 | 92 |
| 4* | 100 | 94 | 79 | 61 | 42 |
| 5 | 100 | 97 | 96 | 100 | 96 |
| 6 | 100 | 100 | 100 | 100 | 100 |
| 7 | 100 | 100 | 92 | 100 | 96 |
| 8 | 100 | 97 | 100 | 100 | 100 |
| 9 | 100 | 100 | 100 | 96 | 100 |
| 10 | 92 | 90.6 | 100 | 81 | 96 |
| 11 | 100 | 100 | 100 | 100 | 100 |
| 12 | 100 | 100 | 96 | 100 | 100 |
Data excluded from statistical analyses of imaging data
Cortical regions
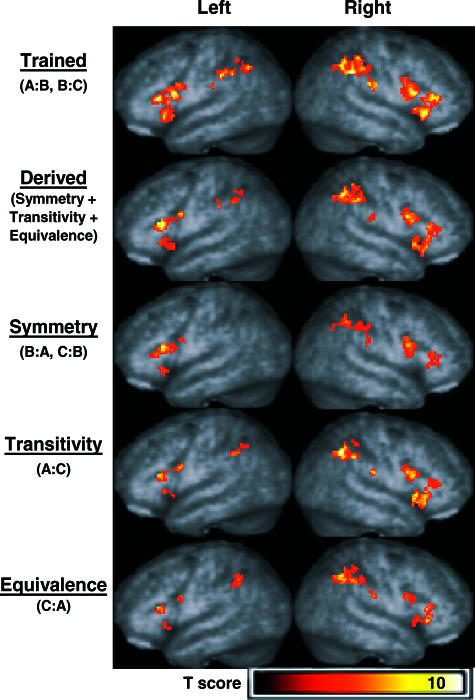
Figure 2 highlights similar activation patterns in frontal and parietal regions for trained and derived relations. Table 2 summarizes the location of voxels with peak activation for each contrast performed along with voxel probabilities, Z scores, and voxel cluster sizes and probabilities. Three-dimensional renderings of areas with activation are shown for the trained relations > match relation contrast (row 1) and for the combined derived relations > match relation contrast (row 2). Conditional responding to trained and derived relations (pooled) produced bilateral activation in ventrolateral and dorsolateral frontal regions and the inferior parietal lobule. Similar activation patterns were observed for the transitivity relations > match relation contrast (row 4) and for the equivalence relations > match relation contrast (row 5). For the symmetry relations > match relation contrast (row 3), activation was similar to all other relations, but absent in the right ventrolateral frontal and left inferior parietal regions.
Fig 2. Activation in frontal and parietal regions of interest exceeding the thresholds p < .005 (uncorrected) and voxel clusters containing more than 15 contiguous voxels.
Three-dimensional renderings of areas with activation appear (from top to bottom, respectively) for the trained relations > match relation contrast, combined derived relations > match relation contrast, symmetry relations > match relation contrast, transitivity relations > match relation contrast, and for equivalence relations > match relation contrast.
Table 2. Regions activated beyond the thresholds of p < .005 with 15 contiguous voxels.
| Relations | Region | Talairach Coordinates |
Voxel Z | Voxel p(unc) | Cluster size | Cluster p(unc) | |||
| x | y | z | |||||||
| Trained (A∶B, B∶C) | Left | Inferior Frontal Gyrus | −55 | 14 | 18 | 4.91 | 0.001* | 636 | 0.001* |
| Inferior Parietal Lobule | −50 | −31 | 38 | 4.69 | 0.001* | 22 | 0.021 | ||
| Right | Inferior Parietal Lobule | 38 | −47 | 39 | 4.98 | 0.001* | 319 | 0.001* | |
| Inferior Frontal Gyrus | 34 | 25 | −6 | 4.52 | 0.001* | 407 | 0.001* | ||
| Derived (Symmetry + Transitivity + Equivalence) | Left | Inferior Frontal Gyrus | −46 | 26 | 15 | 4.56 | 0.001* | 223 | 0.001* |
| Inferior Parietal Lobule | −40 | −45 | 39 | 3.49 | 0.001* | 27 | 0.018 | ||
| Right | Superior Parietal Lobule | 36 | −52 | 50 | 4.21 | 0.001* | 147 | 0.001* | |
| Inferior Frontal Gyrus | 48 | 9 | 25 | 4.13 | 0.001* | 286 | 0.001* | ||
| Inferior Parietal Lobule | 48 | −35 | 48 | 3.44 | 0.001* | 30 | 0.013 | ||
| Symmetry (B∶A, C∶B) | Left | Inferior Frontal Gyrus | −44 | 26 | 17 | 4.66 | 0.001* | 187 | 0.001* |
| Right | Inferior Frontal Gyrus | 50 | 11 | 25 | 3.92 | 0.001* | 263 | 0.001* | |
| Postcentral Parietal Gyrus | 40 | −27 | 46 | 3.70 | 0.001* | 70 | 0.001 | ||
| Inferior Parietal Lobule | 36 | −52 | 50 | 3.64 | 0.001* | 20 | 0.047 | ||
| Transitivity (A∶C) | Left | Inferior Frontal Gyrus | −48 | 9 | 25 | 3.98 | 0.001* | 53 | 0.002 |
| Inferior Parietal Lobule | −38 | −45 | 41 | 3.49 | 0.001* | 25 | 0.022 | ||
| Right | Inferior Parietal Lobule | 36 | −54 | 47 | 4.71 | 0.001* | 102 | 0.001* | |
| Insula | 44 | 16 | 0 | 4.39 | 0.001* | 289 | 0.001* | ||
| Inferior Frontal Gyrus | 48 | 15 | 20 | 4.25 | 0.001* | 210 | 0.001* | ||
| Equivalence (C∶A) | Left | Inferior Frontal Gyrus | −50 | 30 | 17 | 3.98 | 0.001* | 76 | 0.001* |
| Temporal: Angular Gyrus | −36 | −74 | 31 | 3.73 | 0.001* | 17 | 0.057 | ||
| Inferior Parietal Lobule | −36 | −48 | 47 | 3.17 | 0.001 | 42 | 0.005 | ||
| Right | Inferior Frontal Gyrus | 36 | 27 | −3 | 4.47 | 0.001* | 149 | 0.001* | |
| Inferior Parietal Lobule | 36 | −54 | 47 | 3.87 | 0.001* | 136 | 0.001* | ||
| Trained > Derived | Left | Inferior Frontal Gyrus | −38 | 23 | −8 | 3.55 | 0.001* | 83 | 0.001* |
| Right | Inferior Frontal Gyrus | 32 | 25 | −5 | 4.34 | 0.001* | 107 | 0.001* | |
p(unc) = probability uncorrected for multiple comparisons
0.001* = p < .001
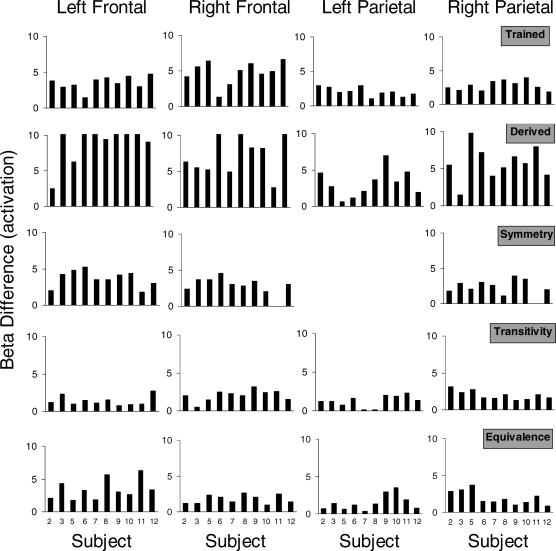
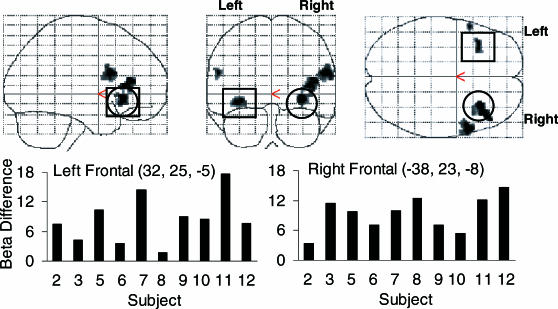
To examine activation for individual subjects, Figure 3 plots beta differences for voxels showing peak activation in left and right frontal and parietal regions displayed in Figure 2. Differences were calculated by subtracting the beta weight (i.e., regression coefficient) for the match relation from the beta weight for each relation individually for each subject. The data displayed are meant to illustrate the consistency of activation across subjects and that group activation patterns displayed in Figure 2 were not averaging artifacts.
Fig 3. Individual subject beta differences for local maxima showing peak activation in left and right frontal and parietal regions of interest.
Reduced threshold analysis
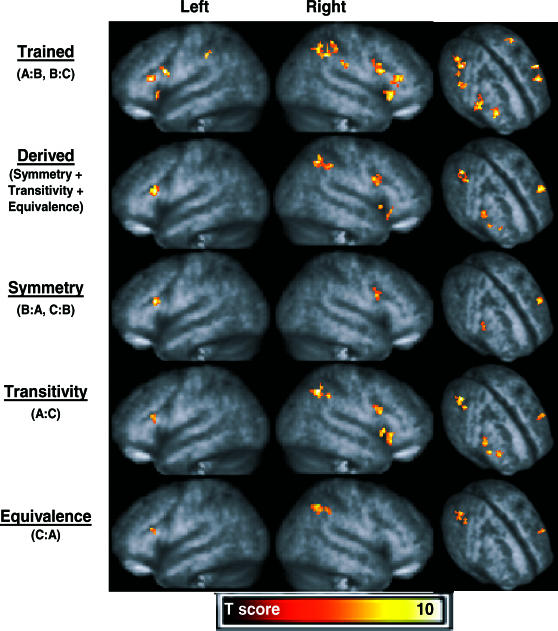
Event-related activity correlated with conditional responding to trained and derived relations was isolated further using a more stringent probability threshold (p < .0005; with an identical extent threshold of 15 contiguous voxels). Figure 4 shows three-dimensional renderings of areas of activation for the trained relations > match relation contrast, combined derived relations > match relation contrast, symmetry relations > match relation contrast, transitivity relations > match relation contrast and for equivalence relations > match relation contrast. Table 3 provides locations of voxels with peak activation for each contrast performed, along with voxel probabilities, Z scores, and voxel cluster sizes and probabilities. A common finding across relations was activation in nearly the same left dorsolateral prefrontal region. In the left cerebrum, however, trained relations also were correlated with ventrolateral frontal and inferior parietal activation. In contrast, considerably more activation was observed in the right cerebrum where the inferior parietal lobule showed activation to trained, derived, transitive, and equivalence relations. The dorsolateral frontal region showed activation to trained, derived, symmetry, and transitive relations. And finally, the ventrolateral frontal region showed activation to trained, derived, and transitive (but not symmetry or equivalence) relations. Such results suggest that potential dissociations exist among derived relations.
Fig 4. Activation in frontal and parietal regions of interest exceeding the thresholds p < .0005 (uncorrected) and voxel clusters containing more than 15 contiguous voxels.
Three-dimensional renderings of areas with activation appear (from top to bottom, respectively) for the trained relations > match relation contrast, combined derived relations > match relation contrast, symmetry relations > match relation contrast, transitivity relations > match relation contrast, and for equivalence relations > match relation contrast.
Table 3. Regions activated beyond the thresholds of p < .0005 with 15 contiguous voxels.
| Relations | Region | Talairach Coordinates |
Voxel Z | Voxel p(unc) | Cluster size | Cluster p(unc) | |||
| x | y | z | |||||||
| Trained (A∶B, B∶C) | Left | Inferior Frontal Gyrus | −55 | 14 | 18 | 4.91 | 0.001* | 51 | 0.001* |
| −44 | 30 | 10 | 4.66 | 0.001* | 58 | 0.001* | |||
| −36 | 23 | −8 | 4.00 | 0.001* | 40 | 0.001* | |||
| Inferior Parietal Lobule | −50 | −31 | 38 | 4.69 | 0.001* | 26 | 0.001* | ||
| Right | Inferior Frontal Gyrus | 34 | 25 | −6 | 4.52 | 0.001* | 92 | 0.001* | |
| 55 | 15 | 20 | 4.49 | 0.001* | 120 | 0.001* | |||
| 44 | 34 | 13 | 4.30 | 0.001* | 70 | 0.001* | |||
| Inferior Parietal Lobule | 38 | −47 | 39 | 4.98 | 0.001* | 99 | 0.001* | ||
| 36 | −38 | 53 | 4.10 | 0.001* | 15 | 0.001 | |||
| 57 | −28 | 31 | 4.03 | 0.001* | 34 | 0.001* | |||
| 53 | −35 | 46 | 3.84 | 0.001* | 26 | 0.001* | |||
| Derived (Symmetry + Transitivity + Equivalence) | Left | Inferior Frontal Gyrus | −46 | 26 | 15 | 4.56 | 0.001* | 74 | 0.001* |
| Right | Inferior Frontal Gyrus | 48 | 9 | 25 | 4.13 | 0.001* | 68 | 0.001* | |
| 44 | 15 | −4 | 3.95 | 0.001* | 15 | 0.003 | |||
| 34 | 27 | −10 | 3.84 | 0.001* | 16 | 0.002 | |||
| Inferior Parietal Lobule | 34 | −54 | 47 | 4.72 | 0.001* | 78 | 0.001* | ||
| Symmetry (B∶A, C∶B) | Left | Inferior Frontal Gyrus | −44 | 26 | 17 | 4.66 | 0.001* | 43 | 0.001* |
| Right | Inferior Frontal Gyrus | 50 | 11 | 25 | 3.92 | 0.001* | 34 | 0.001* | |
| Transitivity (A∶C) | Left | Inferior Frontal Gyrus | −50 | 28 | 17 | 3.96 | 0.001* | 35 | 0.001* |
| Right | Insula | 44 | 16 | 0 | 4.39 | 0.001* | 28 | 0.001* | |
| Inferior Frontal Gyrus | 48 | 15 | 20 | 4.25 | 0.001* | 65 | 0.001* | ||
| 32 | 25 | −11 | 4.06 | 0.001* | 54 | 0.001* | |||
| Inferior Parietal Lobule | 34 | −54 | 47 | 5.33 | 0.001* | 74 | 0.001* | ||
| 38 | −43 | 41 | 4.18 | 0.001* | 21 | 0.001 | |||
| Equivalence (C∶A) | Left | Inferior Frontal Gyrus | −50 | 30 | 17 | 3.98 | 0.001* | 23 | 0.001* |
| Right | Inferior Parietal Lobule | 34 | −54 | 47 | 4.29 | 0.001* | 53 | 0.001* | |
| 36 | −44 | 43 | 3.81 | 0.001* | 18 | 0.001* | |||
p(unc) = probability uncorrected for multiple comparisons
0.001* = p < .001
To explore differences in the extent and magnitude of activation between trained and derived relations, two contrasts were performed using the opposing relation as a comparator condition. Figure 5 presents statistical parametric maps showing activation in inferior frontal regions for the trained relative to derived relations (p < .005, uncorrected, with an extent threshold of 15 contiguous voxels). Bilateral activation appeared in ventrolateral (peak activation highlighted) and dorsolateral prefrontal areas with the extent of activation marginally greater in the right cerebrum. Extraction of individual subject beta differences in voxels with peak activation suggests group results were not averaging artifacts. Collectively, the results suggest that conditional responding to trained and derived relations activate similar regions, with the magnitude of activation being greater for trained relations in frontal regions, and the results from the reduced threshold analysis suggest that derived relations are correlated with dissociable cortical activation patterns.
Fig 5. Statistical parametric maps showing activation in frontal regions of interest for the trained relations > derived relations contrast.
The locations of voxels with peak activation in the left and right cerebrum are highlighted in ventrolateral regions (orbital frontal) and individual subject beta differences displayed. No significant differences were found for the derived relations > trained relations contrast.
Subcortical regions
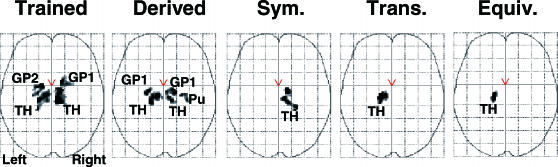
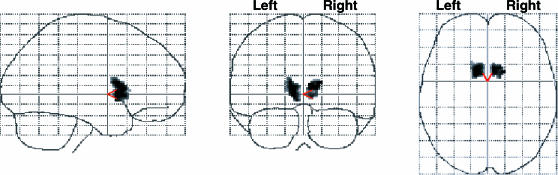
Statistical parametric maps are presented in Figure 6 for trained, pooled derived, and each derived relation from analyses of the thalamus and lentiform nucleus (putamen and globus pallidus). Table 4 provides locations of voxels with peak activation for each contrast performed, along with voxel probabilities, Z scores, and voxel cluster sizes and probabilities. Conditional responding to trained relations was correlated with bilateral activation in the globus pallidus (left medial and right lateral regions) and thalamus, with activation primarily in left ventral lateral and medial dorsal nuclei and right medial dorsal, ventral anterior and ventral lateral nuclei. Mostly similar activation patterns were observed in the globus pallidus and thalamus for derived relations, with the addition of activation in the right putamen. However, activation in thalamic nuclei was focused in the left lateral posterior and ventral lateral nuclei, and right ventral lateral, anterior, and medial dorsal nuclei. When each derived relation was analyzed separately, a different set of activation patterns emerged. Conditional responding to symmetry relations was correlated with activation in the right ventral anterior nucleus and pulvinar. In contrast, activation to transitive relations was localized in the left lateral posterior nucleus, whereas activation to equivalence relations was localized in the ventral lateral nucleus. Direct comparisons between trained and derived relations only revealed activation in the right thalamus (ventral lateral and medial dorsal nuclei) and putamen and left ventral lateral nucleus for trained relations. Similarly, the statistical parametric maps presented in Figure 7 highlight bilateral activation in the caudate head during conditional responding to trained relations relative to the MATCH relation. Similar contrasts performed for derived relations (pooled) and each derived relation did not reveal activation in the caudate. Moreover, contrasts between trained and derived relations revealed greater bilateral caudate activation to trained relations. Collectively, these subcortical analyses suggest that conditional responding to trained and derived relations activate relatively similar subcortical regions, with the exception that trained relations evidence greater caudate and thalamic activation, and along with additional findings suggest that derived relations are correlated with dissociable subcortical activation patterns.
Fig 6. Activation in the thalamus (TH), globus pallidus (GP1 = Lateral Globus Pallidus; GP2 = Medial Globus Pallidus) and putamen (PU) exceeding the thresholds p < .005 and voxel clusters containing more than 15 contiguous voxels.
Statistical parametric maps show activation for the trained relations > match relation contrast, combined derived relations > match relation contrast, symmetry relations > match relation contrast, transitivity relations > match relation contrast, and equivalence relations > match relation contrast. Trained = Relations (A→B, B→C); Derived = Unreinforced relations (Sym+Trans+Equiv); Sym. = Symmetry (B→C, C→B); Trans. = Transitivity (A→C); Equiv. = Equivalence (C→A).
Table 4. Regions activated beyond the thresholds of p < .005 with 15 contiguous voxels.
| Relations | Region | Talairach Coordinates |
Voxel Z | Voxel p(unc) | Cluster size | Cluster p(unc) | |||||
| x | y | z | |||||||||
| Trained (A∶B, B∶C) | Left | Medial | Globus | Pallidus | −14 | 0 | −2 | 3.82 | 0.001* | 97 | 0.001* |
| −18 | −10 | −5 | 3.41 | 0.001* | (97) | ||||||
| Ventral | Lateral | Nucleus | −16 | −17 | 10 | 3.76 | 0.001* | 229 | 0.001* | ||
| Medial | Dorsal | Nucleus | −6 | −11 | 6 | 3.66 | 0.001* | (229) | |||
| Right | Medial | Dorsal | Nucleus | 8 | −17 | 8 | 4.17 | 0.001* | 275 | 0.001* | |
| Ventral | Anterior | Nucleus | 12 | −3 | 11 | 4.12 | 0.001* | (275) | |||
| Ventral | Lateral | Nucleus | 8 | −10 | 4 | 3.63 | 0.001* | (275) | |||
| Lateral | Globus | Pallidus | 14 | 6 | 0 | 4.09 | 0.001* | 76 | 0.001* | ||
| Putamen | 20 | 2 | 9 | 3.29 | 0.001* | (76) | |||||
| Derived (Symmetry + Transitivity + Equivalence) | Left | Lateral | Posterior | Nucleus | −16 | −21 | 12 | 4.14 | 0.001* | 188 | 0.001* |
| Ventral | Lateral | Nucleus | −10 | −13 | 4 | 3.92 | 0.001* | (188) | |||
| Lateral | Globus | Pallidus | −20 | −8 | 4 | 4.06 | 0.001* | 40 | 0.005 | ||
| Right | Ventral | Lateral | Nucleus | 14 | −9 | 13 | 3.74 | 0.001* | 74 | 0.001* | |
| Ventral | Anterior | Nucleus | 10 | −6 | 6 | 2.74 | 0.003 | (74) | |||
| Medial | Dorsal | Nucleus | 10 | −19 | 6 | 3.63 | 0.001* | 62 | 0.001 | ||
| Lateral | Posterior | Nucleus | 16 | −21 | 12 | 2.88 | 0.002 | (62) | |||
| Putamen | 30 | −19 | 8 | 3.42 | 0.001* | 52 | 0.002 | ||||
| Lateral | Globus | Pallidus | 24 | −14 | 0 | 3.38 | 0.001* | (52) | |||
| Putamen | 30 | −20 | −2 | 2.98 | 0.001 | (52) | |||||
| Symmetry (B∶A, C∶B) | Right | Ventral | Anterior | Nucleus | 10 | −6 | 6 | 3.84 | 0.001* | 50 | 0.004 |
| Thalamus | 8 | −17 | 5 | 3.81 | 0.001* | 120 | 0.001* | ||||
| Pulvinar | 12 | −23 | 9 | 3.54 | 0.001* | (120) | |||||
| Transitivity (A∶C) | Left | Thalamus | −10 | −15 | 4 | 4.06 | 0.001* | 136 | 0.001* | ||
| Lateral | Posterior | Nucleus | −16 | −21 | 16 | 3.96 | 0.001* | (136) | |||
| Equivalence (C∶A) | Left | Ventral | Lateral | Nucleus | −14 | −13 | 15 | 3.56 | 0.001* | 41 | 0.006 |
| Trained > Derived | Left | Ventral | Lateral | Nucleus | −10 | −11 | 10 | 3.5 | 0.001* | 60 | 0.001* |
| Right | Ventral | Lateral | Nucleus | 8 | −10 | 4 | 4.18 | 0.001* | 151 | 0.001* | |
| Medial | Dorsal | Nucleus | 8 | −17 | 8 | 4.08 | 0.001* | (151) | |||
| Putamen | 30 | −6 | −3 | 4.17 | 0.001* | 40 | 0.003 | ||||
p(unc) = probability uncorrected for multiple comparisons
0.001* = p < .001
Voxel clusters in parentheses highlight secondary local maxima > 8 mm apart from similar-sized clusters
Fig 7. Statistical parametric maps showing bilateral caudate head activation during discriminations of trained relations (p < .005, extent = 40 contiguous voxels; Left cerebrum: 172 voxels centered at x = −10, y = 10, z = 2, p < .001, Z = 4.30; Right cerebrum: 141 voxels at x = 6, y = 12, z = 8, p < .001, Z = 4.00).
Caudate activation was not observed for derived relations.
Analyses of excluded subjects
Two subjects' data were excluded from the primary analyses (Subjects 1 and 4) because the percentage of correct responses to symmetry, transitive, and equivalence relations did not exceed the performance criterion. These substandard performances provide a unique, but limited, opportunity for determining whether activation was present in our a priori regions for correct responses. Contrasting correct responding to derived relations relative to incorrect responding (correct derived > incorrect derived) provides a marginal test of the prediction that greater activation should be observed if our present findings are accurate. Figure 8 presents three-dimensional renderings of activation in inferior frontal and inferior parietal regions for both subjects. For Subject 1, conditional responding to derived relations (pooled symmetry and transitivity trials) was observed in the left ventrolateral frontal region and bilaterally in inferior parietal regions. Interrogation of subcortical regions revealed activation only in the right lateral globus pallidus (60 voxels; p = .008). For Subject 4, whose performance was more accurate than Subject 1, bilateral activation was observed in ventral lateral frontal and parietal regions and the left dorsal lateral frontal region. The extent of activation also appears somewhat greater relative to Subject 1. Although suggestive of a trend consistent with our prediction, these findings must be viewed cautiously because individual analyses are not comparable to group analyses (e.g., a small number of correct trials was used, variability is based across scans, and lenient thresholds were employed).
Fig 8. Three-dimensional renderings of activation in inferior frontal and inferior parietal regions for 2 subjects excluded from the group analyses (top: Subject 1; bottom: Subject 4).
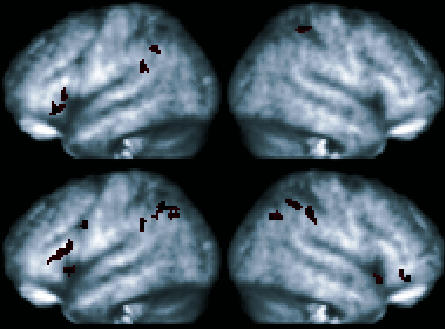
Results show significantly greater activation (p < .05, extent = 20 contiguous voxels) for correct responding to derived relations (pooled) as compared to incorrect responding to derived relations.
Discussion
In this investigation, correct responding on MTS trials containing trained and derived relations was correlated with bilateral activation in the inferior parietal lobule and dorsolateral and ventrolateral inferior frontal regions, as well as in portions of the thalamus and globus pallidus. Such similarities may not be surprising given the cascade of similar behavioral responses involved during matching-to-sample, which includes attending and remembering the sample stimulus, identifying a correct comparison based upon past contingencies, and emitting a spatially oriented response (Dickins, 2005). Collectively, the extent of common activation observed for both trained and derived relations suggests that the targeted frontal-subcortical and frontal-parietal regions support conditional responding to trained relations established through reinforcement and the derived relations that arise from reinforced conditional relations. Finally, we found that group results obtained with conventional neuroimaging approaches, with subjects treated as a random factor, appear to reasonably reflect individual subject results.
The inferior parietal activation observed is consistent with results reported in numerous human imaging studies that involve the remembering of previously observed stimuli and stimulus relations, and that manipulate new information during transitive inference (e.g., Acuna et al., 2002; Habeck et al, 2005; Lepage et al., 2003; Prince, Daselaar, & Cabeza, 2005; Shannon & Buckner, 2004). Similarly, activation in dorsolateral frontal regions is consistent with decision-making functions of this region highlighted in studies on declarative memory, SE, temporally extended choice behavior, and transitive inference (Acuna et al.; Barnes-Holmes et al., 2005; Dickins et al., 2001; Shallice, 2002; Yarkoni, Braver, Gray, & Green, 2005). The consistent left dorsolateral activation observed across relations also may reflect successful recognition of visual relations (Prince et al., 2005), whereas ventrolateral prefrontal activation may suggest working memory involvement during the period extending from sample presentation until choice. However, although ventral lesions impair recognition following long delays, extended training facilitates relearning of conditional relations suggesting that ventrolateral prefrontal cortex activation is not essential for working memory (Rushworth, Nixon, Eacott, & Passingham, 1997). Instead, ventral prefrontal cortex activity may simply reflect incoming sensory information, suggesting that this region responds regardless of whether a conditional relation is present, and the magnitude of the response may increase with an increase in sensory load. Significant differences in activation in prefrontal regions and the caudate for trained relations relative to derived relations may reflect the effects of prolonged training which serves to solidify the integration of stimulus, response, and outcome relations. Alternatively, activation may reflect upcoming performance feedback much like reward-predicting cues that increase neural activity in various medial and ventral frontal regions and the caudate (e.g., Knutson, Fong, Bennett, Adams, & Hommer, 2003; Tremblay & Schultz, 2000).
One notable discrepancy between the present investigation and Dickins et al. (2001) was our failure to replicate a left lateralized effect. We observed predominantly right cerebral activation for trained and derived relations, as well as individually for transitive and equivalence relations, at reduced thresholds suggesting that conditional responding within the MTS context is mediated more by nonverbal than verbal processes (Walter et al., 2003). Specifically, right dorsolateral frontal and inferior parietal activation may reflect visuospatial demands imposed by the stimulus arrangement on MTS trials and the spatial response requirement (Colvin, Handy, & Gazzaniga, 2003; Vallar, Bottini, & Paulesu, 2003). However, our use of MATCH trials presumably would have recruited the same visuospatial and visuomotor processes as trained and derived trials, consequently ‘subtracting’ these elements from our final results. An equally plausible, related account of the right lateralized outcome stems from the serial ordering of stimuli within classes established through sequential A, B, and C training. Consequently, discrimination of correct class-consistent comparisons within the trained array may recruit spatial memory processes originating in right inferior frontal and parietal regions (Smith, Jonides, & Koeppe, 1996) or behavior related to relational framing, with A viewed as being ‘before’ B and C, and C as being ‘after’ A and B. One rather simple approach for assessing the contributions of visuospatial and visuomotor demands in SE research would be to employ auditory or tactile stimuli. Employing different training procedures that vary the order in which stimulus relations are established within classes (e.g., one-to-many or many-to-one approaches; Saunders & Green, 1999) is yet another strategy available to examine the effects of training history on subsequent activation.
There are a number of other differences between our findings and those reported by Dickens et al. (2001). These authors reported activation in the inferior parietal region that appears in more posterior regions than ours (perhaps in BA 7, 19, and 39; also see Dickins, 2005), which may reflect increased saccadic eye movements produced by having to consecutively view multiple comparison stimuli (Bisley & Goldberg, 2003). Dickins et al. also reported activation in the left caudate for trained and derived relations and in the right thalamus for trained and equivalence relations, whereas we found bilateral caudate activation for trained relations, bilateral thalamic activation for trained and pooled derived relations, and different unilateral thalamic activation across derived relations. Presumably, some of these discrepancies are methodologically based (trained relations not initially reinforced, block presentations of MTS trials during imaging) whereas others may reflect different imaging analyses (block versus our event-related approach). Not only will investigations be needed to isolate these differences, but the functional contributions of other regions known to contribute to conditional learning will need exploration, such as the roles of the cerebellum, hippocampal system, inferior and superior temporal cortex, and premotor areas (Passingham et al., 2000; Takeda, Naya, Fujimichi, Takeuchi, & Miyashita, 2005).
After neuroimaging many of our subjects offered unsolicited descriptions of the strategy they used to remember stimulus relations. Although such strategies may have been prompted by our instructions to learn relations among stimuli, this seems unlikely given that Dickins et al. (2001) reported similar findings using different instructions. Nevertheless, one reported strategy involved visualizing a linear sequence of stimuli within classes (e.g., A1→B1→C1). Another involved explicit naming (e.g., CAT, FROG, CAR). For some subjects, names of stimuli were semantically related—with stimuli in both classes given animal names. For others, names between stimulus classes were semantically different—with one class denoted by animal names and the other class denoted by object names. One subject combined naming with a storyline [“the CAT (A1) met the FROG (B1) to go driving in the CAR (C1)”]. Although we did not systematically collect data on such strategies and we recognize the complexities and shortcomings associated with quantifying post hoc verbal reports, nonetheless, strategies are difficult behaviors to ignore, particularly in neuroimaging research where different strategies will recruit anatomically different regions. We did find it especially revealing that most reports appeared to parallel the order of stimuli within classes (A1→B1, B1→C1). Whether serial ordering accounts for the present results is unclear, but it does suggest functional relationships exist among training order, strategies, and the unique activation patterns observed for derived relations.
A comment seems warranted on neuroimaging and cognitive subtraction. First, it seems important to recognize that our results were obtained relative to one type of sensorimotor control task. Our rationale for employing matching two identical circles as a comparator condition was to reduce the likelihood of activation appearing in the end results due to motor processes (eye and hand movements), motor–spatial mapping, visual–spatial mapping, and attentional shifts. It may seem obvious, but the t tests utilized to identify activation in our experimental conditions relative to the sensory-control condition were just one tool for identifying significant differences in parameter estimates (i.e., BOLD signal)—and, as shown here and elsewhere, a welcome approach for identifying individual subject effects (Schlund & Cataldo, 2005). Performing voxel-wise contrasts with t-tests (or any inferential test) and using BOLD measures does not, however, reflect a commitment to cognitive subtraction. We recognize that performing contrasts can never be theoretically neutral; after all, subtraction is not performed by tools but rather it is a theoretical approach performed by investigators. Often, it seems to us, the tools and the logic of subtraction are criticized in the same breath—effectively tossing out the baby with the bathwater. Failure to distinguish between tools and subtraction overshadows serious efforts to effectively discover how neuroimaging can be used effectively at the intersection of the experimental analysis of behavior and neuroscience. Minimizing assumptions about underlying processing mechanisms and focusing on behavior seems a good start. In addition, we propose exploring other experimental designs and contrast arrangements, particularly ones that facilitate experimental control over regional activation. One approach involves imaging before and after baseline training and using pretraining imaging data as the sensorimotor control condition to assess training-related changes in activation. Embedding multiple control conditions in designs to isolate regions responding to stimulus novelty or number of comparisons are other approaches for systematically revealing regional sensitivity. Because SE is a product of learning history and MTS performance involves a number of different, sequentially ordered behaviors, it is our belief that a comprehensive account of the neurobiology of SE, and derived knowledge more generally, will emerge through innovative neuroimaging designs.
Acknowledgments
Research and manuscript preparation were partly supported by NIH Grant R03 HD43178-01A1 awarded to the first author.
Appendix
Blocks of trained relations completed within each session and percent correct responses for Subjects 1–12.
| Subject | Session | Trials | Match | Blocks of Trained Relations |
|||||
| A1B1 | A2B2 | Mixed AB | B1C1 | B2C2 | Mixed BC | ||||
| 1 | 1 | 25 | 100 | 33 | 83 | 75 | — | — | — |
| 2 | 25 | 100 | 100 | 100 | 100 | — | — | — | |
| 3 | 25 | 100 | — | — | — | 100 | 50 | 87 | |
| 4 | 25 | 100 | — | — | — | 83 | 100 | 75 | |
| 5 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 6 | 25 | 100 | — | — | 80 | — | — | 90 | |
| 7 | 25 | 100 | — | — | 80 | — | — | 100 | |
| 8 | 25 | 100 | — | — | 80 | — | — | 90 | |
| 9 | 25 | 100 | — | — | 80 | — | — | 100 | |
| 10 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 2 | 1 | 25 | 100 | 50 | 100 | 100 | — | — | — |
| 2 | 25 | 100 | 100 | 83 | 100 | — | — | — | |
| 3 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 4 | 25 | 100 | — | — | 90 | — | — | 100 | |
| 5 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 3 | 1 | 25 | 100 | 83 | 83 | 100 | — | — | — |
| 2 | 25 | 100 | 83 | 83 | 87.5 | — | — | — | |
| 3 | 25 | 100 | 100 | 100 | 100 | — | — | — | |
| 4 | 25 | 100 | — | — | — | 83 | 100 | 100 | |
| 5 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 6 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 7 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 4 | 1 | 25 | 100 | 16 | 100 | 50 | — | — | — |
| 2 | 25 | 100 | 16 | 100 | 63 | — | — | — | |
| 3 | 25 | 100 | 33 | 100 | 87 | — | — | — | |
| 4 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 5 | 25 | 100 | — | — | 50 | — | — | 50 | |
| 6 | 25 | 100 | — | — | 60 | — | — | 70 | |
| 7 | 25 | 100 | — | — | 100 | — | — | 80 | |
| 8 | 25 | 100 | — | — | 80 | — | — | 100 | |
| 9 | 25 | 100 | — | — | 70 | — | — | 100 | |
| 10 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 5 | 1 | 25 | 100 | 100 | 100 | 100 | — | — | — |
| 2 | 25 | 100 | 100 | 100 | 100 | — | — | — | |
| 3 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 4 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 5 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 6 | 1 | 25 | 100 | 83 | 100 | 100 | — | — | — |
| 2 | 25 | 100 | 100 | 100 | 100 | — | — | — | |
| 3 | 25 | 100 | — | — | — | 83 | 100 | 100 | |
| 4 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 5 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 6 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 7 | 1 | 25 | 100 | 83 | 100 | 100 | — | — | — |
| 2 | 25 | 100 | 100 | 100 | 100 | — | — | — | |
| 3 | 25 | 100 | — | — | — | 83 | 100 | 100 | |
| 4 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 5 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 6 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 8 | 1 | 25 | 100 | 0 | 67 | 88 | — | — | — |
| 2 | 25 | 100 | 100 | 100 | 100 | — | — | — | |
| 3 | 25 | 100 | — | — | — | 67 | 100 | 100 | |
| 4 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 5 | 25 | 100 | — | — | 80 | — | — | 100 | |
| 6 | 25 | 100 | — | — | 90 | — | — | 90 | |
| 7 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 8 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 9 | 1 | 25 | 100 | 100 | 100 | 100 | — | — | — |
| 2 | 25 | 100 | — | — | — | 83 | 100 | 100 | |
| 3 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 4 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 5 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 10 | 1 | 25 | 100 | 67 | 100 | 100 | — | — | — |
| 2 | 25 | 100 | 100 | 100 | 100 | — | — | — | |
| 3 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 4 | 25 | 100 | — | — | 90 | — | — | 100 | |
| 5 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 6 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 11 | 1 | 25 | 100 | 100 | 100 | 100 | — | — | — |
| 2 | 25 | 100 | 100 | 100 | 100 | — | — | — | |
| 3 | 25 | 100 | — | — | — | 83 | 100 | 100 | |
| 4 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 5 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 6 | 25 | 100 | — | — | 100 | — | — | 100 | |
| 12 | 1 | 25 | 100 | 66 | 83 | 87 | — | — | |
| 2 | 25 | 100 | 100 | 100 | 87 | — | — | — | |
| 3 | 25 | 100 | 100 | 100 | 100 | — | — | — | |
| 4 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 5 | 25 | 100 | — | — | — | 100 | 100 | 100 | |
| 6 | 25 | 100 | — | — | 100 | — | — | 100 | |
References
- Acuna B.D, Eliassen J.C, Donoghue J.P, Sanes J.N. Frontal and parietal lobe activation during transitive inference in humans. Cerebral Cortex. 2002;12:1312–1321. doi: 10.1093/cercor/12.12.1312. [DOI] [PubMed] [Google Scholar]
- Barnes-Holmes D, Regan D, Barnes-Holmes Y, Commins S, Walsh D, Stewart I, Smeets P.M, Whelan R, Dymond S. Related derived relations as a model of analogical reasoning: reaction times and event-related potentials. Journal of the Experimental Analysis of Behavior. 2005;84:435–451. doi: 10.1901/jeab.2005.79-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley J.W, Goldberg M.E. The role of the parietal cortex in the neural processing of saccadic eye movements. In: Siegel A.M, Andersen R.A, Freund H, Spenser D.D, editors. Advances in neurology: Vol. 93. The parietal lobes. Philadelphia: Lippincott Williams & Wilkins, Maple-Press; 2003. pp. 141–158. [PubMed] [Google Scholar]
- Brett M. The MNI brain and the talairach atlas. 2002. [Google Scholar]
- Brasted P.J, Wise S.P. Comparison of learning-related neuronal activity in the dorsal premotor cortex and striatum. European Journal of Neuroscience. 2004;19:721–740. doi: 10.1111/j.0953-816x.2003.03181.x. [DOI] [PubMed] [Google Scholar]
- Bussey T.J, Wise S.P, Murray E.A. Interaction of ventral and orbital prefrontal cortex with inferotemporal cortex in conditional visuomotor learning. Behavioral Neuroscience. 2002;116:703–715. [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Colvin M.K, Handy T.C, Gazzaniga M.S. Hemispheric asymmetries in the parietal lobes. In: Siegel A.M, Andersen R.A, Freund H, Spenser D.D, editors. Advances in neurology: Vol. 93. The parietal lobes. Philadelphia: Lippincott Williams & Wilkins, Maple-Press; 2003. pp. 321–334. [PubMed] [Google Scholar]
- Cowley B.J, Green G, Braunling-McMorrow D. Using stimulus equivalence procedures to teach name-face matching to adults with brain injuries. Journal of Applied Behavior Analysis. 1992;25:461–475. doi: 10.1901/jaba.1992.25-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonet J.F, Thierry G, Cardebat D. Renewal of the neurophysiology of language: Functional neuroimaging. Physiological Reviews. 2005;85:49–95. doi: 10.1152/physrev.00049.2003. [DOI] [PubMed] [Google Scholar]
- Devany J.M, Hayes S.C, Nelson R.O. Equivalence class formation in language-able and language-disabled children. Journal of the Experimental Analysis of Behavior. 1986;46:243–257. doi: 10.1901/jeab.1986.46-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickins D.W. On aims and methods in the neuroimaging of derived relations. Journal of the Experimental Analysis of Behavior. 2005;84:453–483. doi: 10.1901/jeab.2005.92-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickins D.W, Singh K.D, Roberts N, Burns P, Downes J.J, Jimmieson P, Bentall R.P.B. An fMRI study of stimulus equivalence. Neuroreport. 2001;12:405–411. doi: 10.1097/00001756-200102120-00043. [DOI] [PubMed] [Google Scholar]
- Eacott M.J, Gaffan D. Inferotemporal-frontal disconnections: The uncinate fascicle and visual associative learning in monkey. European Journal of Neuroscience. 1992;4:1320–1332. doi: 10.1111/j.1460-9568.1992.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan R.J. Differential neural responses during performance of matching and nonmatching to sample tasks at two different delay intervals. The Journal of Neuroscience. 1999;19:5066–5073. doi: 10.1523/JNEUROSCI.19-12-05066.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Uecker A. 2005. Talairch daemon client [Computer software]. [Google Scholar]
- Friman P.C, Hayes S.C, Wilson K.G. Why behavior analysts should study emotion: The example of anxiety. Journal of Applied Behavior Analysis. 1998;31:137–156. doi: 10.1901/jaba.1998.31-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J, Ashburner J, Frith C.D, Poline J.B, Heather J.D, Frackowiak R.S.J. Spatial registration and normalization of images. Human Brain Mapping. 1995;3:165–189. [Google Scholar]
- Friston K.J, Worsley K.J, Frackowiak R.S.J. Statistical parametric maps in functional imaging: A general linear approach [Abstract]. Human Brain Mapping. 1995;2:189. [Google Scholar]
- Gaffen D, Harrison S. Auditory-visual associations, hemispheric specialization and temporal-frontal interaction in the rhesus monkey. Brain. 1991;114:2133–2144. doi: 10.1093/brain/114.5.2133. [DOI] [PubMed] [Google Scholar]
- Grossman M, Smith E.E, Koenig P, Glosser G, DeVita C, Moore P, McMillan C. The neural basis for categorization in semantic memory. Neuroimage. 2002;17:1549–1561. doi: 10.1006/nimg.2002.1273. [DOI] [PubMed] [Google Scholar]
- Habeck C, Rakitin B.C, Moeller J, Scarmeas N, Zarahn E, Brown T, Stern Y. An event related fMRI study of the neural networks underlying the encoding, maintenance and retrieval phase in a delayed-match-to-sample task. Cognitive Brain Research. 2005;23:207–220. doi: 10.1016/j.cogbrainres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Hayes S.C, Barnes-Holmes D, Roche B. Relational frame theory: A post-Skinnerian account of language and cognition. New York: Kulver Academic/Plenum Publishers; 2001. [DOI] [PubMed] [Google Scholar]
- Holmes A.P, Friston K.J. Generalisability, random effects and population inference [Abstract]. NeuroImage: Abstracts of the 4th International Conference on Functional Mapping of the Human Brain. 1998;7:S754, 136. [Google Scholar]
- Inase M, Li B.M, Takashima I, Iijima T. Pallidal activity is involved in visuomotor association learning in monkeys. European Journal of Neuroscience. 2001;14:897–901. doi: 10.1046/j.0953-816x.2001.01701.x. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong G.W, Bennett S.M, Adams C.M, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event-related fMRI. NeuroImage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Lepage M, Brodeur M, Bourgouin P. Prefrontal cortex contribution to associative recognition memory in humans: an event related functional magnetic imaging study. Neuroscience Letters. 2003;346:73–76. doi: 10.1016/s0304-3940(03)00578-0. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A, Laurienti P.J, Burdette J.H, KraftA R. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McDermott K.B, Jones T.C, Peterson S.E, Lageman S.K, Roediger H.L. Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: An event related fMRI study. Journal of Cognitive Neuroscience. 2000;12:965–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- Melchiori L.E, de Souza D.G, de Rose J.C. Reading, equivalence, and recombination of units: A replication with students with different learning histories. Journal of Applied Behavior Analysis. 2000;33:97–100. doi: 10.1901/jaba.2000.33-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miezin F.M, Maccotta L, Ollinger J.M, Petersen S.E, Buckner R.L. Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Manning F.J. Nonspatial memory after selective prefrontal lesions in monkeys. Brain Research. 1978;143:313–323. doi: 10.1016/0006-8993(78)90571-1. [DOI] [PubMed] [Google Scholar]
- Moerk E.L. An archeology of meaning. Journal of the Experimental Analysis of Behavior. 1997;68:248–251. doi: 10.1901/jeab.1997.68-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E.A, Gaffen D, Mishkin M. Neural substrates of visual stimulus-stimulus association in rhesus monkeys. The Journal of Neuroscience. 1993;13:4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B, Friederici A.D. Interactions of the hippocampal system and the prefrontal cortex in learning language-like rules. NeuroImage. 2003;19:1730–1737. doi: 10.1016/s1053-8119(03)00170-8. [DOI] [PubMed] [Google Scholar]
- Passingham R.E. Delayed matching after selective prefrontal lesions in monkeys. Brain Research. 1975;92:89–102. doi: 10.1016/0006-8993(75)90529-6. [DOI] [PubMed] [Google Scholar]
- Passingham R.E, Toni I, Rushworth M.F.S. Specialisation within the prefrontal cortex: the ventral prefrontal cortex and associative learning. Experimental Brain Research. 2000;133:103–113. doi: 10.1007/s002210000405. [DOI] [PubMed] [Google Scholar]
- Plaud J.J, Gaither G.A, Weller L.A, Bigwood S.J, Barth J, von Duvillard S.P. Rational-emotive behavior therapy and the formation of stimulus equivalence classes. Journal of Clinical Psychology. 1998;54:597–610. doi: 10.1002/(sici)1097-4679(199808)54:5<597::aid-jclp6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Porter M.C, Koch J, Mair R.G. Effects of reversible inactivation of thalamo-striatal circuitry on delayed matching trained with retractable levers. Behavioural Brain Research. 2001;119:61–69. doi: 10.1016/s0166-4328(00)00331-4. [DOI] [PubMed] [Google Scholar]
- Prince S.E, Daselaar S.M, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. Journal of Neuroscience. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci P.T, Zelkowicz B.J, Nebes R.D, Meltzer C.C, Mintun M.A, Becker J.T. Functional neuroanatomy of semantic memory: Recognition of semantic associations. NeuroImage. 1999;9:88–96. doi: 10.1006/nimg.1998.0386. [DOI] [PubMed] [Google Scholar]
- Rushworth M, Nixon P.D, Eacott M.J, Passingham R.E. Ventral prefrontal cortex is not essential for working memory. Journal of Neuroscience. 1997;17:4829–4838. doi: 10.1523/JNEUROSCI.17-12-04829.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders K.J, O'Donnell J, Vaidya M, Williams D.C. Recombinative generalization of within-syllable units in nonreading adults with mental retardation. Journal of Applied Behavior Analysis. 2003;36:95–99. doi: 10.1901/jaba.2003.36-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders R.R, Drake K.M, Spradlin J.E. Equivalence class establishment, expansion, and modification in preschool children. Journal of the Experimental Analysis of Behavior. 1999;71:195–214. doi: 10.1901/jeab.1999.71-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders R.R, Green G. A discrimination analysis of training-structure effects on stimulus equivalence outcomes. Journal of the Experimental Analysis of Behavior. 1999;72:117–137. doi: 10.1901/jeab.1999.72-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlund M, Pace G, McGready J. Relations between decision-making deficits and discriminating contingencies following brain injury. Brain Injury. 2001;15:1061–1071. doi: 10.1080/02699050110086887. [DOI] [PubMed] [Google Scholar]
- Schlund M.W, Cataldo M.C. Integrating functional neuroimaging and human operant research: brain activation correlated with presentation of discriminative stimuli. Journal of the Experimental Analysis of Behavior. 2005;84:505–519. doi: 10.1901/jeab.2005.89-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlund M.W, Pace G. The effects of traumatic brain injury on reporting and responding to causal relations: An investigation of sensitivity to reinforcement contingencies. Brain Injury. 2000;14:573–583. doi: 10.1080/026990500120475. [DOI] [PubMed] [Google Scholar]
- Shallice T. Fractionation of the supervisory system. In: Stuss D.T, Knight R.T, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 261–277. [Google Scholar]
- Shannon B.J, Buckner R.L. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. Journal of Neuroscience. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M. Equivalence relations and the reinforcement contingency. Journal of the Experimental Analysis of Behavior. 2000;74:127–146. doi: 10.1901/jeab.2000.74-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.E, Jonides J, Koeppe R.A. Dissociating verbal and spatial working memory using PET. Cerebral Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Takeda M, Naya Y, Fujimichi R, Takeuchi D, Miyashita Y. Active maintenance of associative mnemonic signal in monkey inferior temporal cortex. Neuron. 2005;48:839–848. doi: 10.1016/j.neuron.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Reward-related neuronal activity during go-nogo task performance in primate orbitofrontal cortex. Journal of Neurophysiology. 2000;83:1864–1876. doi: 10.1152/jn.2000.83.4.1864. [DOI] [PubMed] [Google Scholar]
- Toni I, Ramnani N, Josephs O, Ashburner J, Passingham R.E. Learning arbitrary visuomotor associations: temporal dynamics of brain activity. NeuroImage. 2001;14:1048–1057. doi: 10.1006/nimg.2001.0894. [DOI] [PubMed] [Google Scholar]
- Tonneau F, Arreola F, Martinez A.G. Function transformation without reinforcement. Journal of the Experimental Analysis of Behavior. 2006;85:393–405. doi: 10.1901/jeab.2006.49-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Bottini G, Paulesu E. Neglect syndromes: The role of the parietal cortex. In: Siegel A.M, Andersen R.A, Freund H, Spenser D.D, editors. Advances in neurology: Vol. 93. The parietal lobes. Philadelphia: Lippincott Williams & Wilkins, Maple-Press; 2003. pp. 293–320. [PubMed] [Google Scholar]
- Walter H, Bretschneider V, Gron G, Zurowski B, Wunderlich A.P, Tomczak R, Spitzer M. Evidence for quantitative domain dominance for verbal and spatial working memory in frontal and parietal cortex. Cortex. 2003;39:897–911. doi: 10.1016/s0010-9452(08)70869-4. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Braver T.S, Gray J.R, Green L. Prefrontal brain activity predicts temporally extended decision-making behavior. Journal of the Experimental Analysis of Behavior. 2005;84:537–554. doi: 10.1901/jeab.2005.121-04. [DOI] [PMC free article] [PubMed] [Google Scholar]