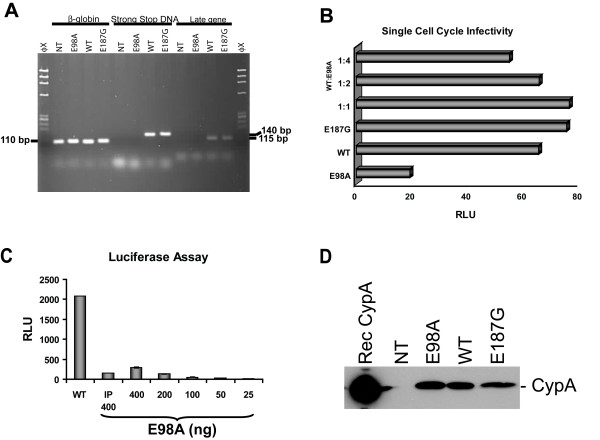
Figure 3.
Viral infectivity assay. (A) Detection of proviral DNA. H9 cells were infected as above and total cellular DNA was prepared 16 days post-infection using Qiagen's DNA isolation kit and analyzed by PCR using a set of primers specific for negative strand strong-stop DNA and a conserved region of the gag, described previously [30, 31]. Early gene products were amplified using the forward primer Ra 5'-TCT CTG GTT AGA CCA GAT CTG-3' (459–479) and the reverse primer U5a 5'-GTC TGA GGG ATC TCT AGT TAC-3' (584–604). Late gene products representing a conserved region of the HIV-1 gag was amplified with the forward primer SK-38 5'-ATC CAC CTA TCC CAG TAG GAG AAA T-3' (1090–1117) and the reverse primer SK-39 5'-TTT GGT CCT TGT CTT ATG TCC AGA ATG C-3' (1177–1204) that amplified a 115-bp fragment. We also examined the viral cDNA production at 16 hrs post-infection and been able to detect in all cells infected with mutant and wild-type virions (data not shown). To normalize for the quantity of total cellular DNA present in each sample, human β-globin DNA was amplified [29]. (B) Single cell cycle infectivity of mutant and wild-type virus particles on TZM-bl reporter cell lines. Cells (2 × 104) were infected as described above with equal amounts (25 ng p24 antigen) of mutant and wild-type virus or chimeric virus stock prepared by co-transfection of mutant and wild-type pNL4-3 at a ratio of 1:1, 2:1, and 4:1. Infected cells were then cultured in the presence of 5 μM indinavir. Twenty-four hours post-infection, cells were harvested in 200 μl Glo lysis buffer (Promega) and assayed for luciferase activity with the luciferase assay kit obtained from Promega. RLU, relative light unit. (C) TZM-bl cells (8 × 104) were infected as described above with 400 ng of wild-type NL4-3 virus or with E98A virus that was first immunoprecipitated with anti-Tat monoclonal antibody (indicated with IP 400). Cells were also infected with E98A virus stock that had been two-fold serially diluted. After 48 hrs, culture supernatants were removed and cells were assayed for luciferase activity. (D) Detection of virion associated cyclophilin A (CypA) by Western blot analysis. Cell free culture supernatants from 293T cells transfected with mutant and wild-type pNL4-3 were equilibrated for p24 antigen concentration and equal amounts of virus was precipitated with Viraffinity (CPG Inc) as recommended by the manufacturer. Culture supernatants were mixed (4:1) with Viraffinity and the mixture was incubated at room temperature for 5 min and centrifuged at 1000 × g for 10 min. The viral pellets were washed and dissolved in 1× RIPA buffer [50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate and 0.1% SDS, supplemented with a complete protease inhibitor cocktail from Roche]. The viral proteins were finally separated by SDS-PAGE, transferred onto a nitrocellulose membrane and probed with rabbit anti-CypA antibody (Calbiochem, 1:2,000) and as secondary antibody horseradish peroxidase-conjugated anti-rabbit IgG. Rec CypA, recombinant CypA; NT, non-transfected control.