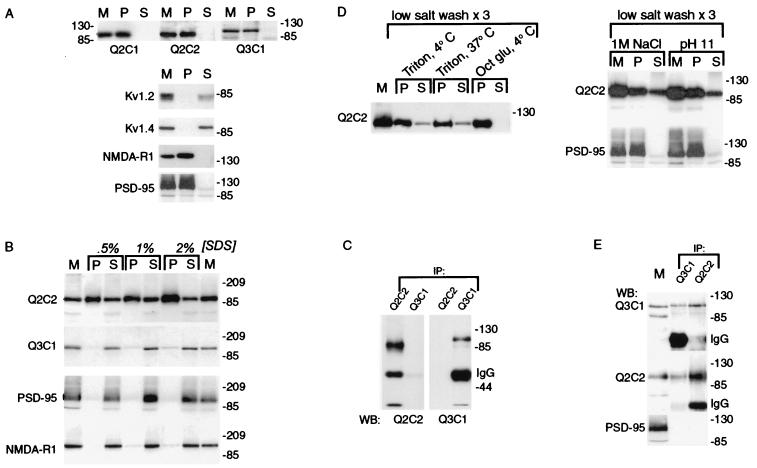
Figure 3.
Heteromeric human brain KCNQ2/KCNQ3 channels are contained within a Triton X-100-resistant subcellular fraction. (A) Insolubility of KCNQ2 and KCNQ3. Human cortical membranes were solubilized in 1% Triton X-100, and soluble and insoluble fractions were separated by centrifugation. Starting membranes (M) and insoluble (P, pellet) and soluble (S) fractions were probed with antibodies against KCNQ subunits, Shaker family potassium channel subunits (Kv1.2, Kvl.4), a glutamate receptor subunit (NMDA-R1), and PSD-95. The Shaker subunits were efficiently solubilized by Triton X-100; the KCNQ subunits, NMDA-R1 and PSD-95 were poorly solubilized. (B) Solubilization by SDS at 4°C. Human brain membranes were solubilized with SDS at the indicated concentrations on ice as described in Materials and Methods, and soluble and insoluble fractions were separated by centrifugation. KCNQ3, NMDA-R1, and PSD-95 controls were efficiently solubilized by cold SDS, but the majority of KCNQ2 remained associated with the insoluble fraction. (C) SDS-solubilized KCNQ2 and KCNQ3 are not coassociated. After solubilization of brain membranes using 1% SDS/4°C, Q2C1 and Q3C2 immunoprecipitates were probed with the indicated antibodies. (D) Solubilization of KCNQ2/KCNQ3 complexes (but not PSD-95) by Triton X-100 after low salt washes, high salt, and pH 11. Membranes were washed as described in Materials and Methods, then solubilized as indicated with octyl glucoside or Triton X-100 at 4°C or 37°C as indicated. KCNQ2 and KCNQ3 (not shown) were partly solubilized by Triton X-100 after low-salt washes, sequential low/high-salt washes, or pH 11, but not by octyl glucoside, and solubilization with Triton X-100 at 37°C after low-salt washes was not more effective than at 4°C. PSD-95 remained in pellet after salt or pH 11 treatment. (E) Coimmunoprecipitation of KCNQ2 and KCNQ3 by either Q2C2 or Q3C1 antibodies. Membranes were low-salt washed three times, extracted at pH 11, solubilized with Triton X-100, and cleared of insoluble material by centrifugation. KCNQ2 and KCNQ3 (but not PSD-95) were both immunoprecipitated by antibodies specific for either subunit.