Abstract
In this study, we have used a technique designed to target short fragments containing informative mitochondrial substitutions to extend the temporal limits of DNA recovery and study the molecular phylogeny of Ursus deningeri. We present a cladistic analysis using DNA recovered from 400 kyr old U. deningeri remains, which demonstrates U. deningeri's relation to Ursus spelaeus. This study extends the limits of recovery from skeletal remains by almost 300 kyr. Plant material from permafrost environments has yielded DNA of this age in earlier studies, and our data suggest that DNA in teeth from cave environments may be equally well preserved.
Keywords: evolution, Ursidae, ancient DNA, Atapuerca
1. Introduction
When DNA from prehistoric tissue was introduced as a genetic source material to the scientific community, a possibility to study evolutionary processes seemed realistic. Attempts were also made to extract DNA from geological material (Cano et al. 1993; Woodward et al. 1994), and contemporary authentication criteria seemed to support some of these results (Bada et al. 1994). However, when higher stringency was applied to these types of study, it became evident that several of the oldest DNA sequences were based on contaminating DNA (Zischler et al. 1995; Austin et al. 1997). Most ancient DNA sequences that have been thoroughly authenticated are less than 100 000 years, with only a few exceptions (Loreille et al. 2001; Willerslev et al. 2003). The number of copies should increase with decreased fragment size, as DNA in ancient tissue is degraded (Smith et al. 2003; Noonan et al. 2005). Therefore, it may be possible to extend the age limit of amplifiable DNA by targeting short fragments. Further, by selecting specific informative point mutations, it may be possible to apply evolutionary questions to DNA from fossils of great age. We investigate this possibility here by extracting and amplifying specific polymorphic sites in cytochrome b from bear material (Ursus deningeri) excavated from the Atapuerca cave complex in northern Spain, a site from which younger prehistoric material has yielded DNA (Anderung et al. 2005).
Ursus deningeri was a part of the Pleistocene European Ursidae family represented by two main evolutionary lineages, one resulting in the extant brown bear (Ursus arctos) and the other leading to the cave bear (Ursus spelaeus). These two lineages diverged in Eurasia about 1.2 million years ago (Loreille et al. 2001), and their common ancestor has been suggested to be the so-called Etruscan bear, Ursus etruscus (Soergel 1912). Ursus dolinensis has been recently suggested to represent the earliest member of the speleoid lineage (García & Arsuaga 2001). Ursus deningeri is first recognized in the European fossil record at around 600 kyr ago, and lasts until the Late Middle Pleistocene (García et al. 1997) when U. spelaeus appears. This latter species went extinct at approximately 12 kyr (BP) (Kurtén 1968). Cave bears are thus believed to have evolved as a single, unbroken lineage U. dolinensis–U. deningeri–U. spelaeus (García & Arsuaga 2001). To investigate the possibility of using molecular data from Middle Pleistocene mammals, we have focused on the genetic relationship between U. deningeri and U. spelaeus.
2. Material and methods
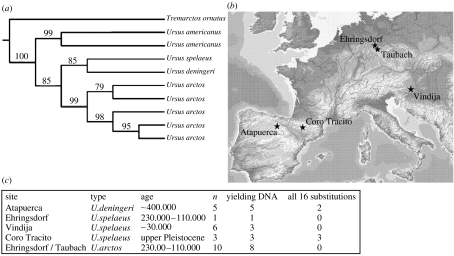
A total of 25 bear samples from five different well-studied Pleistocene fossil sites in Europe were analysed (figure 1). The U. deningeri samples (n=5) were recovered from the Sima de los Huesos site in the Sierra de Atapuerca (García et al. 1997), and date to approximately 400 kyr (Bischoff et al. 2003). The site is located at a depth of 25 m below the present ground surface, with conditions of constant humidity and temperature (11 °C). The U. spelaeus samples analysed were recovered from: (i) Late Middle Pleistocene (ca 230 kyr old) or Early Upper Pleistocene (ca 120 kyr old) travertines of Ehringsdorf (Weimar), Central Germany (n=1; Kurtén 1975; Kahlke et al. 2002); (ii) 30-kyr-old sediments of Vindija cave, Croatia (n=6; Smith et al. 1999); and (iii) the undated Upper Pleistocene site of Coro Tracito cave, Pyrenees, Spain (n=3; Torres et al. 1998). In addition, a set of U. arctos samples, originating from the Ehringsdorf (Weimar) travertine sequence (from ca 220–110 kyr) and the last interglacial travertine sands of Taubach (Weimar; n=10; Kurtén 1977) of around 120 kyr, was analysed.
Figure 1.
(a) Phylogenetic position of Ursus deningeri. Maximum parsimony tree using molecular data from cytochrome b. The grouping of U. deningeri and U. spelaeus was well supported (1000 replicates). (b) Sites for the caves with bear samples. (c) Out of 25 samples, 20 yielded bear DNA, out of which 5 yielded all 16 polymorphic sites.
DNA in prehistoric material is present in minute amounts and highly fragmented (Hofreiter et al. 2001). Since the probability of amplifying short fragments of DNA is likely to be higher than the amplifying longer fragments (Smith et al. 2003; Noonan et al. 2005), genetic markers were selected to be short and specific for bear mitochondrial polymorphisms from the cytochrome b gene that could differentiate among three different species of ursids: U. arctos, U. spelaeus and U. americanus. As we aimed for minimal amplicon size, primer sites were always less than 6 bp upstream and downstream from the polymorphic site. DNA was extracted from the material with a phosphate buffer, and purified and concentrated with a hybridization/magnetic separation technique (Anderung et al. 2005). The DNA was then amplified and typed with pyrosequencing technology.
3. Results
A total of 20 individuals yielded DNA (see electronic supplementary material) and five of them yielded all the 16-targeted polymorphisms in cytochrome b. From two of the oldest samples, 400 kyr old U. deningeri (Bischoff et al. 2003), we were able to pyrosequence all 16 polymorphisms (the remaining three yielded DNA, but not all targeted sites). Seven of the U. spelaeus samples yielded DNA, three of them yielded the same information from cytochrome b as U. deningeri or more. For the Ehringsdorf and Taubach U. arctos samples, we were able to pyrosequence up to 13 polymorphic sites. When the genetic data were compared with previously published Ursus sp. sequences, the phylogenetic tree gave strong support for the placement of U. deningeri together with U. spelaeus (figure 1).
4. Discussion
Owing to the non-overlapping chronological ranges of U. deningeri and U. spelaeus, there are two possible hypotheses regarding the evolutionary relationship between them. First, they could be sister species, that diverged about 600 kyr ago. Second, U. deningeri could be the direct ancestor of U. spelaeus. Obtaining DNA sequences from both species using samples collected close to the transition period should allow these two hypotheses to be tested. Although U. deningeri and U. spelaeus had identical DNA sequences, it is likely that the total sequence length (41 bp) obtained in this study is insufficient to resolve these two hypotheses. Additional sequence data including more polymorphic sites, e.g. variable within U. spelaeus, would be needed.
Authentication followed the standard rules (Cooper & Poinar 2000). The work was carried out in a new ancient DNA laboratory, where no DNA work (ancient or modern) had been undertaken. Replication was carried out in an ancient DNA laboratory, where there had been no previous work with bear DNA. When the replication was carried out, ancient samples of elephant (Loxodonta africana) and lemming (Lemmus sibiricus) were included as negative controls. These did not produce bear sequences, but did give appropriate results when targeted with appropriate species-specific primers. Further, the U. deningeri samples did not produce amplicons with the elephant- and lemming-specific primers. A non-random degradation pattern has been suggested to be a problem in ancient DNA (Gilbert et al. 2003; Banerjee & Brown 2004). The similarity between recent and the ca 220–110 kyr old U. arctos in this study indicates that the sequences obtained from U. deningeri are not non-randomly degraded U. arctos fragments. Since the minimal fragment sizes targeted could not be efficiently quantified with real-time PCR (real-time PCR techniques demand larger fragments), we based sequences and reactions on a minimum of three extractions (with the exception for some of the younger U. arctos that were only extracted twice).
Targeting short DNA fragments appears to be a powerful tool when working with highly degraded DNA. Using this technique, we were able to retrieve genetic information from 400 kyr old U. deningeri samples. Typing polymorphic sites in short mitochondrial fragments may yield access to genetic data on other Middle Pleistocene animals in a similar fashion.
Acknowledgments
We want to thank Ian Barnes, Ignacio Canudo, Juan Echevarria, Hans Ellegren, F. Clark Howell, Ignacio Martínez, Carles Víla, Niklas Wahlberg, Tim White and Rolf Quam for their various contributions to this study. DGVI 1253/03, BOS2003-08938-C03-01, MECyD and CSIC provided financial support.
Footnotes
Present addresses: Department of Archaeology, University of Durham, Durham DH1 3LE, UK and Department of Human Evolution, Max Planck Institute for Evolutionary Anthropology, Deutscher Platz 6, 04103 Leipzig, Germany.
References
- Anderung C, et al. Prehistoric contacts over the Straits of Gibraltar indicated by genetic analysis of Iberian Bronze Age cattle. Proc. Natl Acad. Sci. USA. 2005;102:8431–8435. doi: 10.1073/pnas.0503396102. doi:10.1073/pnas.0503396102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J.J, Ross A.J, Smith A.B, Fortey R.A, Thomas R.H. Problems of reproducibility—does geologically ancient DNA survive in amber-preserved insects? Proc. R. Soc. B. 1997;264:467–474. doi: 10.1098/rspb.1997.0067. doi:10.1098/rspb.1997.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada J.L, Wang X.S, Poinar H.N, Paabo S, Poinar G.O. Amino acid racemization in amber-entombed insects: implications for DNA preservation. Geochim. Cosmochim. Acta. 1994;58:3131–3135. doi: 10.1016/0016-7037(94)90185-6. doi:10.1016/0016-7037(94)90185-6 [DOI] [PubMed] [Google Scholar]
- Banerjee M, Brown T. Non-random DNA damage resulting from heat treatment: implications for sequence analysis of ancient DNA. J. Archaeol. Sci. 2004;31:59–63. doi:10.1016/S0305-4403(03)00099-2 [Google Scholar]
- Bischoff J.L, Shamp D.D, Aramburu A, Arsuaga J.L, Carbonell E, Bermudez de Castro J.M. The Sima de los Huesos hominids date to beyond U/Th equilibrium (>350 kyr) and perhaps to 400–500 kyr: new radiometric dates. J. Archaeol. Sci. 2003;30:275–280. doi:10.1006/jasc.2002.0834 [Google Scholar]
- Cano R.J, Poinar H.N, Pieniazek N.J, Acra A, Poinar G.O., Jr Amplification and sequencing of DNA from a 120–135-million-year-old weevil. Nature. 1993;363:536–538. doi: 10.1038/363536a0. doi:10.1038/363536a0 [DOI] [PubMed] [Google Scholar]
- Cooper A, Poinar H.N. Ancient DNA: do it right or not at all. Science. 2000;289:1139. doi: 10.1126/science.289.5482.1139b. doi:10.1126/science.289.5482.1139b [DOI] [PubMed] [Google Scholar]
- García N, Arsuaga J.L. Ursus dolinensis: a new species of Early Pleistocene ursid from Trinchera Dolina, Atapuerca (Spain) Comptes Rendus de l'Académie des Sciences de Paris, série II. 2001;332:717–725. [Google Scholar]
- García N, Arsuaga J.L, Torres T. The carnivore remains from the Sima de los Huesos Middle Pleistocene site (Sierra de Atapuerca, Spain) J. Hum. Evol. 1997;33:155–174. doi: 10.1006/jhev.1997.0154. [DOI] [PubMed] [Google Scholar]
- Gilbert M.T, Willerslev E, Hansen A.J, Barnes I, Rudbeck L, Lynnerup N, Cooper A. Distribution patterns of postmortem damage in human mitochondrial DNA. Am. J. Hum. Genet. 2003;72:32–47. doi: 10.1086/345378. doi:10.1086/345378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofreiter M, Serre D, Poinar H.N, Kuch M, Paabo S. Ancient DNA. Nat. Rev. Genet. 2001;2:353–359. doi: 10.1038/35072071. doi:10.1038/35072071 [DOI] [PubMed] [Google Scholar]
- Kahlke R.-D, Maul L.C, Meyrick R.A, Stebich M, Grasselt T. The quaternary sequence from the late Middle to Upper Pleistocene site of Weimar–Ehringsdorf. In: Meyrick R.A, Schereve D.C, editors. The quaternary of central Germany (Thuringia and surroundings) Quaternary Research Association; London, UK: 2002. pp. 163–177. [Google Scholar]
- Kurtén B. Weidenfeld & Nicolson; London, UK: 1968. Pleistocene mammals of Europe. [Google Scholar]
- Kurtén B. Fossile Reste von Hyänen und Bären (Carnivora) aus den Travertinen von Weimar-Ehringsdorf. In: Kahlke H.-D, editor. Das Pleistozän von Weimar-Ehringsdorf. Teil 2. Abh. Zentr. Geol. Inst., Paläont. Abh. vol. 23. Akademie-Verlag; Berlin, Germany: 1975. pp. 465–484. [Google Scholar]
- Kurtén B. Bären—und Hyänenreste aus dem Pleistozän von Taubach. In: Kahlke H.-D, editor. Das Pleistozän von Taubach bei Weimar. Quartärpaläontologie. vol. 2. Akademie-Verlag; Berlin, Germany: 1977. pp. 361–378. [Google Scholar]
- Loreille O, Orlando L, Patou-Mathis M, Philippe M, Taberlet P, Hanni C. Ancient DNA analysis reveals divergence of the cave bear, Ursus spelaeus, and brown bear, Ursus arctos, lineages. Curr. Biol. 2001;11:200–203. doi: 10.1016/s0960-9822(01)00046-x. doi:10.1016/S0960-9822(01)00046-X [DOI] [PubMed] [Google Scholar]
- Noonan J.P, et al. Genomic sequencing of Pleistocene cave bears. Science. 2005;309:597–599. doi: 10.1126/science.1113485. doi:10.1126/science.1113485 [DOI] [PubMed] [Google Scholar]
- Smith F.H, Trinkaus E, Pettitt P.B, Karavanic I, Paunovic M. Direct radiocarbon dates for Vindija G1 and Velika Pecina Late Pleistocene hominid remains. Proc. Natl Acad. Sci. USA. 1999;96:12 281–12 286. doi: 10.1073/pnas.96.22.12281. doi:10.1073/pnas.96.22.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.I, Chamberlain A.T, Riley M.S, Stringer C, Collins M.J. The thermal history of human fossils and the likelihood of successful DNA amplification. J. Hum. Evol. 2003;45:203–217. doi: 10.1016/s0047-2484(03)00106-4. doi:10.1016/S0047-2484(03)00106-4 [DOI] [PubMed] [Google Scholar]
- Soergel W. Gustav Fischer; Jena, Germany: 1912. Das Aussterben diluvialer Säugetiere und die Jagd des diluvialen Menschen. [Google Scholar]
- Torres T, Canudo J.I, Cobo R, Cuenca G. Cueva Coro Tracito (Tella Sin, Huesca) el primer yacimiento de alta montaña Español de Ursus spelaeus Ros.-Hein. Nota Preliminar. Geogaceta. 1998;24:303–306. [Google Scholar]
- Willerslev E, et al. Diverse plant and animal genetic records from Holocene and Pleistocene sediments. Science. 2003;300:791–795. doi: 10.1126/science.1084114. doi:10.1126/science.1084114 [DOI] [PubMed] [Google Scholar]
- Woodward S.R, Weyand N.J, Bunnell M. DNA sequence from Cretaceous period bone fragments. Science. 1994;266:1229–1232. doi: 10.1126/science.7973705. [DOI] [PubMed] [Google Scholar]
- Zischler H, Hoss M, Handt O, von Haeseler A, van der Kuyl A.C, Goudsmit J. Detecting dinosaur DNA. Science. 1995;268:1192–1193. doi: 10.1126/science.7605504. [DOI] [PubMed] [Google Scholar]