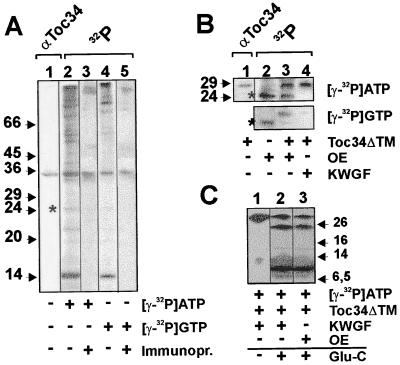
Figure 2.
Toc34 can be phosphorylated with ATP and GTP by a kinase present in the outer envelope or by a kinase-enriched fraction from wheat germ lysate. (A) Outer envelope membranes were subjected to SDS/PAGE and immunodecorated with αToc34 antibodies (lane 1). Outer envelope membranes (20 μg of total protein) were incubated with [γ-32P]ATP (lanes 2 and 3) or [γ-32P]GTP (lanes 4 and 5) and analyzed on SDS/PAGE before (lanes 2 and 4) and after immunoprecipitation using αToc34 antibody (lanes 3 and 5). (B) Outer envelopes (10 μg of total protein; OE) were incubated with γ-32P-labeled nucleotides in the absence (lane 2) or presence (lane 3) of 2 μg of purified Toc34ΔTM252–6His. The immunodecoration of the mixture using αToc34 antibodies is shown in lane 1 for identification of Toc34ΔTM252–6His. In lane 4, purified Toc34ΔTM252–6His (2 μg) and 0.5 ng of a protein kinase-enriched wheat germ fraction (KWGF) (15) were incubated with [γ-32P]ATP or [γ-32P]GTP. (A and B) A phosphorylated unknown 24-kDa protein is indicated for comparison of labeling efficiency (*). (C) Toc34ΔTM252–6His phosphorylated by the protein kinase-enriched wheat germ fraction (lanes 1 and 2, KWGF) or a kinase present in the outer envelope (lane 3) was cleaved by using endoproteinase Glu-C. Arrows and numbers in A–C indicate the molecular mass standard in kDa.