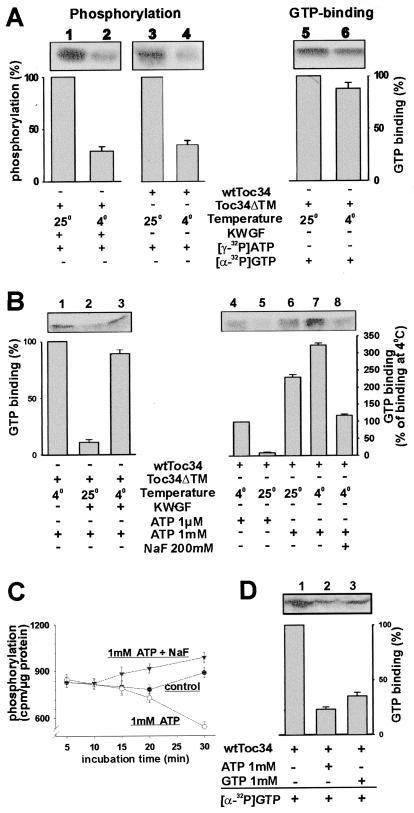
Figure 3.
Phosphorylation of Toc34 is temperature-dependent and regulates GTP binding to Toc34. (A) wtToc34 (lanes 3 and 4) was phosphorylated by a protein kinase present in outer envelopes and Toc34ΔTM252–6His (2 μg; lanes 1 and 2) by a protein kinase-enriched wheat germ fraction (0.5 ng total protein, KWGF) in the presence of [γ-32P]ATP at 25°C (lanes 1 and 3) or 4°C (lanes 2 and 4). Equal amounts of Toc34ΔTM252–6His were incubated with [α-32P]GTP at 25°C (lane 5) or 4°C (lane 6), and GTP binding was determined. (B) A total of 2 μg of purified Toc34ΔTM252–6His was incubated for 20 min with [α-32P]GTP and 1 mM ATP in the absence (lane 1) or presence (lanes 2 and 3) of the protein kinase-enriched wheat germ fraction (0.5 ng total protein, KWGF) at 4°C (lanes 1 and 3) or 25°C (lane 2), and binding of GTP was determined. A total of 20 μg of outer envelope protein was incubated for 20 min with [α-32P]GTP in the presence of 1 μM ATP (lanes 4 and 5) or 1 mM ATP (lanes 6–8) at 25°C (lanes 5 and 6) or 4°C (lanes 4, 7, and 8). In lane 7, 200 mM of NaF was added. (C) Phosphorylation of wtToc34 present in outer envelopes by [γ-32P]ATP (●) in the presence of 1 mM ATP (○) or 1 mM ATP and 200 mM NaF (▾) was determined at different incubation times. Each point represents the average of at least two independent experiments. (D) Outer envelopes were phosphorylated for 5 min with either 1 mM ATP (lane 2) or 1 mM GTP (lane 3), washed twice without nucleotides and then incubated with [α-32P]GTP. (A, B, and D) The averaged results of at least three independent experiments are shown as histogram. (A) The observed phosphorylation or GTP binding at 25°C (lanes 1, 3, and 5); (B) the GTP binding at 4°C (lanes 1 and 4); and (D) the GTP binding without phosphorylation was set to 100%.