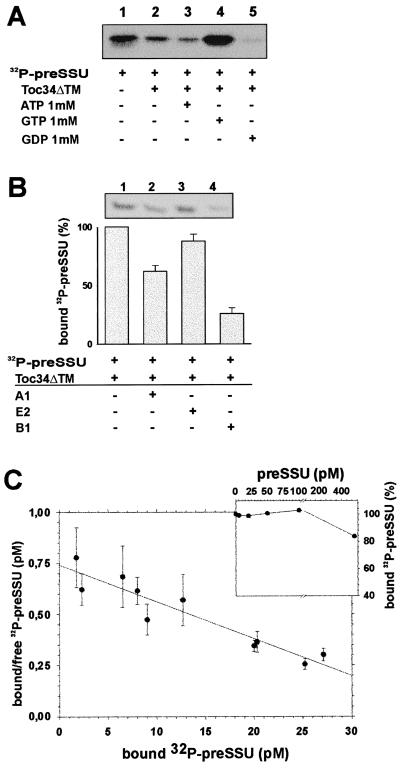
Figure 4.
Toc34 interacts specifically with the phosphorylated precursor protein preSSU. (A) A total of 20 pM of [32P]preSSU (25% shown in lane 1) was incubated with 10 nM of Toc34ΔTM252–6His (lanes 2–5) in the presence of 1 mM ATP (lane 3), 1 mM GTP (lane 4), and 1 mM GDP (lane 5), and the complex was immunoprecipitated by using αToc34 antibodies. (B) A total of 20 pM [32P]preSSU were incubated with 10 nM Toc34ΔTM252–6His in the presence of 1 mM GTP (lane 1). After 10 min, 250 nM of nonphosphorylated peptide A1 (MVAPFTGLKSAASFPVSRKQNLDITS; lane 2), E2 (MASSVLSSAAVATRSN VAQAN; lane 3), or 250 nM phosphorylated peptide B1 (MVAPFTGLKSA AS*FPVSRKQNLDITS; lane 4) was added, and the remaining complex was immunoprecipitated. Histogram represents the average of at least three independent experiments, where [32P]preSSU binding to Toc34ΔTM252–6His in the absence of competitor was set to 100%. (C) A total of 0.1 nM Toc34ΔTM252–6His was incubated with different amounts of [32P]preSSU in the presence of 1 mM GTP, the complex was immunoprecipitated, and the binding was quantified. The average of three independent experiments is shown as Skatchard plot, resulting in a dissociation constant of Kd = 0.10 ± 0.04 nM; C Inset) A total of 0.1 nM Toc34ΔTM252–6His was incubated with 10 pM (●) [32P]preSSU in presence of 1 mM GTP and of different amounts of nonphosphorylated preSSU. Values are expressed as percent [32P]preSSU bound without competition with nonphosphorylated preSSU.