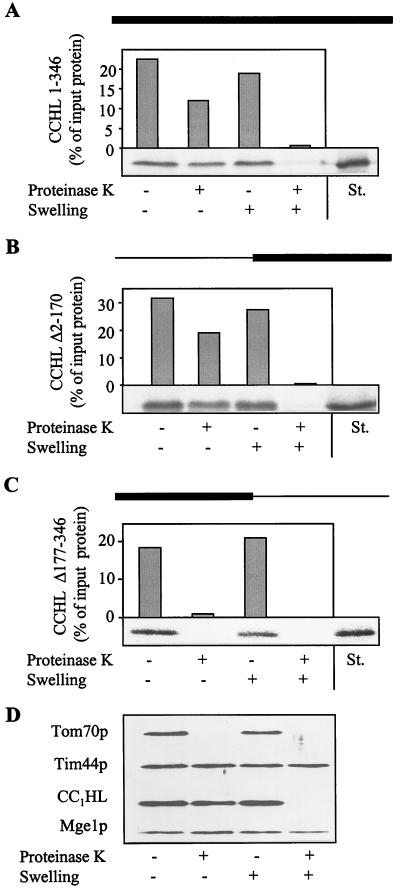
Figure 1.
CCHL does not contain essential import information in its N-terminal half. The precursor proteins CCHL (A), CCHL Δ2–170 (B), and CCHL Δ177–346 (C; see Materials and Methods) were synthesized by in vitro transcription and translation in reticulocyte lysate by using [35S]methionine as a label. The black bar on top of each panel represents the region of CCHL present in these proteins; the thin line corresponds to deleted segments. The radiolabeled proteins were added to isolated mitochondria in import buffer (36). After incubation for 10 min at 25°C, mitochondria were reisolated by centrifugation (10 min at 9,000 × g). Mitochondria were resuspended in a small volume of SoH buffer (0.6 M sorbitol/20 mM Hepes-KOH, pH 7.2). Samples were diluted 10-fold into SoH buffer or water in the presence or absence of proteinase K as indicated. The hypotonic condition results in swelling of the organelles and leads to selective rupture of the outer membrane allowing added proteinase K to degrade proteins exposed in the intermembrane space (D, CC1HL) but not of proteins of the matrix (Tim44p and Mge1p). After incubation for 30 min at 0°C, protease digestion was halted by the addition of PMSF, and proteins were precipitated with trichloroacetic acid. Proteins were separated by SDS/PAGE, blotted on nitrocellulose, and quantified by PhosphorImager analysis. In addition, an autoradiograph is shown. The material that is not digested after swelling represents aggregated preprotein. The rightmost lanes contain 50% of input preprotein as a standard (St.). Import (i.e., protease-resistant protein relative to bound material) varied by not more than 15% in various experiments.