Abstract
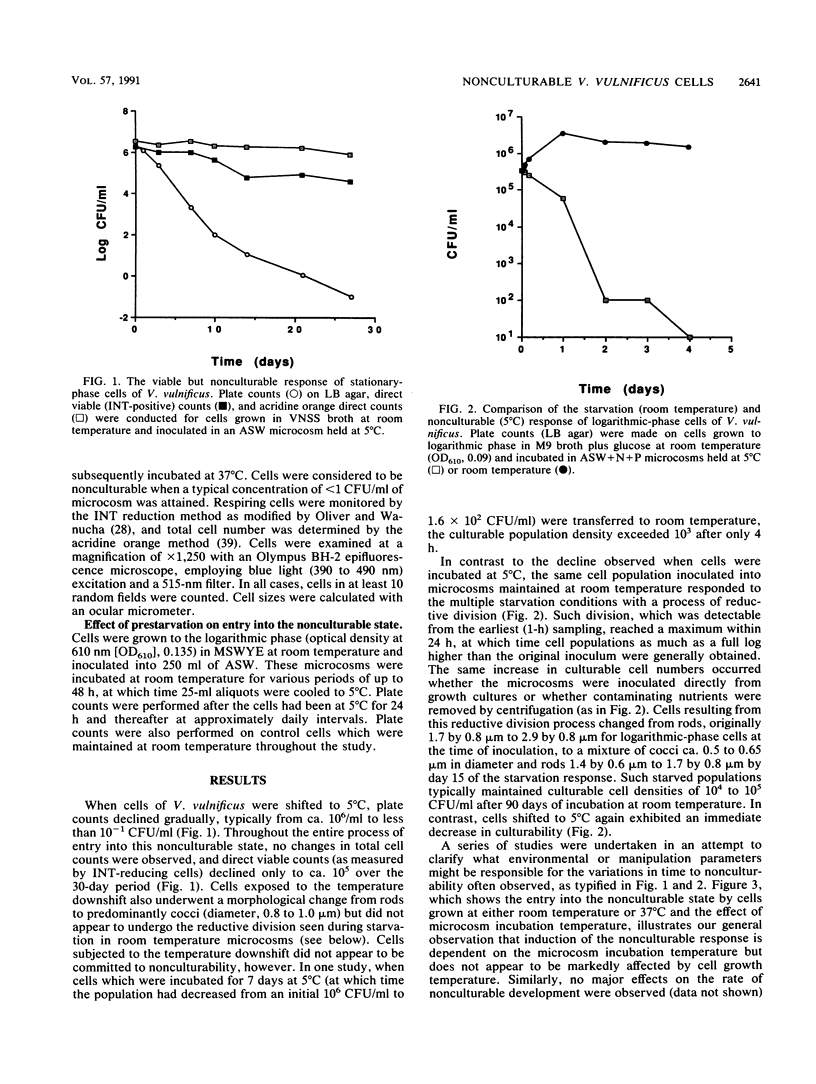
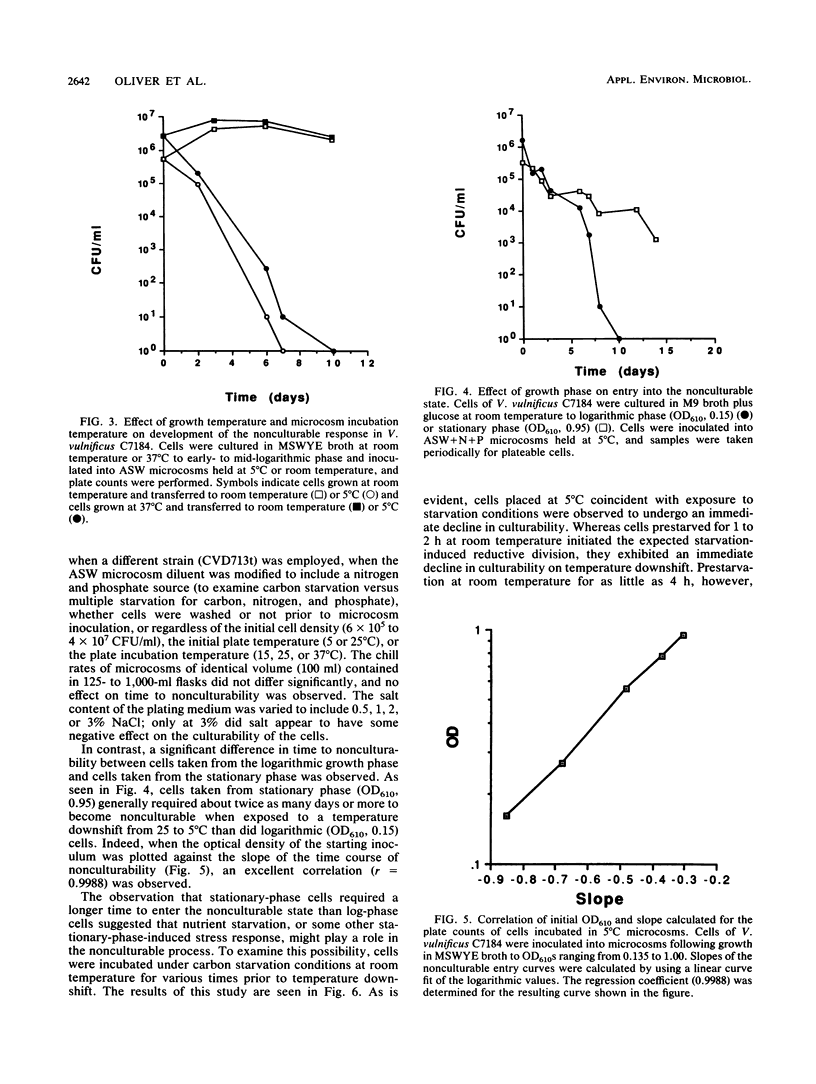
Entry into the viable but nonculturable state by the human bacterial pathogen Vibrio vulnificus in artificial seawater microcosms was studied. In contrast to the long-term culturability exhibited by cells incubated under these starvation conditions at room temperature, cells exposed to a temperature downshift to 5 degrees C exhibited an immediate decrease in culturability. Cells incubated at low temperature exhibited a morphological change from rods to cocci but demonstrated no reductive division. Of 10 factors studied which might affect the nonculturable response in V. vulnificus, only the physiological age of the cells was found to significantly affect the rate at which cells became nonculturable. The nonculturable response appears to be related to the starvation response, as prestarvation at room temperature for 24 h was found to eliminate the nonculturable response of cells subsequently incubated at 5 degrees C. This observation suggests that the synthesis of starvation proteins may repress the viable but nonculturable program displayed during low-temperature incubation. The possible ecological significance of these findings is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broeze R. J., Solomon C. J., Pope D. H. Effects of low temperature on in vivo and in vitro protein synthesis in Escherichia coli and Pseudomonas fluorescens. J Bacteriol. 1978 Jun;134(3):861–874. doi: 10.1128/jb.134.3.861-874.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J. J., Colwell R. R. Maintenance of plasmids pBR322 and pUC8 in nonculturable Escherichia coli in the marine environment. Appl Environ Microbiol. 1990 Jul;56(7):2104–2107. doi: 10.1128/aem.56.7.2104-2107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M. A., Yamanaka H., Miyoshi S., Aziz K. M., Shinoda S. Ecology of Vibrio mimicus in aquatic environments. Appl Environ Microbiol. 1989 Aug;55(8):2073–2078. doi: 10.1128/aem.55.8.2073-2078.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J., Pollitt N. S., Inouye M. Major cold shock protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(1):283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff K. A. Survival of Vibrio anguillarum and Vibrio salmonicida at different salinities. Appl Environ Microbiol. 1989 Jul;55(7):1775–1786. doi: 10.1128/aem.55.7.1775-1786.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. G., VanBogelen R. A., Neidhardt F. C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987 May;169(5):2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysner C. A., Abeyta C., Jr, Wekell M. M., DePaola A., Jr, Stott R. F., Leitch J. M. Virulent strains of Vibrio vulnificus isolated from estuaries of the United States West Coast. Appl Environ Microbiol. 1987 Jun;53(6):1349–1351. doi: 10.1128/aem.53.6.1349-1351.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl Environ Microbiol. 1982 Oct;44(4):820–824. doi: 10.1128/aem.44.4.820-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M., Mårdén P., Jones G. W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- Lange R., Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Linder K., Oliver J. D. Membrane fatty acid and virulence changes in the viable but nonculturable state of Vibrio vulnificus. Appl Environ Microbiol. 1989 Nov;55(11):2837–2842. doi: 10.1128/aem.55.11.2837-2842.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- Nilsson L., Oliver J. D., Kjelleberg S. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J Bacteriol. 1991 Aug;173(16):5054–5059. doi: 10.1128/jb.173.16.5054-5059.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T., Flärdh K., Kjelleberg S. Responses to multiple-nutrient starvation in marine Vibrio sp. strain CCUG 15956. J Bacteriol. 1990 Dec;172(12):7085–7097. doi: 10.1128/jb.172.12.7085-7097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill K. R., Jones S. H., Grimes D. J. Incidence of Vibrio vulnificus in northern New England water and shellfish. FEMS Microbiol Lett. 1990 Oct;60(1-2):163–167. doi: 10.1016/0378-1097(90)90365-w. [DOI] [PubMed] [Google Scholar]
- Oliver J. D., Warner R. A., Cleland D. R. Distribution and ecology of Vibrio vulnificus and other lactose-fermenting marine vibrios in coastal waters of the southeastern United States. Appl Environ Microbiol. 1982 Dec;44(6):1404–1414. doi: 10.1128/aem.44.6.1404-1414.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Warner R. A., Cleland D. R. Distribution of Vibrio vulnificus and other lactose-fermenting vibrios in the marine environment. Appl Environ Microbiol. 1983 Mar;45(3):985–998. doi: 10.1128/aem.45.3.985-998.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Rollins D. M., Colwell R. R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986 Sep;52(3):531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Simpson L. M., White V. K., Zane S. F., Oliver J. D. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect Immun. 1987 Jan;55(1):269–272. doi: 10.1128/iai.55.1.269-272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Almirón M., Kolter R. surA, an Escherichia coli gene essential for survival in stationary phase. J Bacteriol. 1990 Aug;172(8):4339–4347. doi: 10.1128/jb.172.8.4339-4347.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


