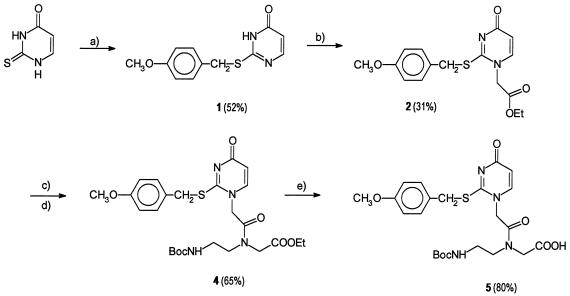
Scheme 1.
Synthesis of 2-thiouracil PNA monomer. (a) 4-Methoxybenzyl chloride in alkaline (KOH) water/EtOH (1:1). (b) Ethyl bromoacetate in ethanolic sodium ethoxide followed by precipitation from ethyl acetate. (1H nuclear Overhauser enhancement NMR analysis and comparison of the 13C NMR spectrum with reports in the literature of N-alkylated S-benzylthiouracils (13) confirmed the assignment of the desired product (N1-alkylation.) (c) LiOH in water/MeOH/tetrahydrofuran (THF) (1:3:7). (d) Ethyl N-(2-Boc-aminoethyl)glycinate/O-benzotriazole-1-yl-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) in dimethylformamide. (e) LiOH in water/MeOH/THF (1:3:7).