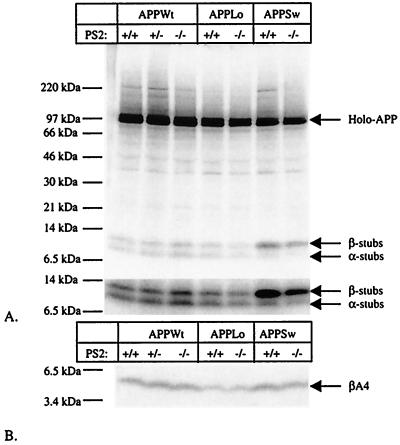
Figure 5.
APP processing in neurons derived from E14 PS2−/− embryos. (A) Analysis of APP expression and generation of α- and β-secretase-generated APP carboxyterminal stubs (23, 38). Neurons were infected with recombinant Semliki Forest Virus driving expression of human wild-type APP (APPWt) or APP containing the London (APPLo) or the Swedish (APPSw) type of AD-causing mutations. After metabolic labeling, antibody B12/4 recognizing the 20 carboxyterminal amino acid residues of APP was used to immunoprecipitate holo-APP and α- and β-secretase-generated carboxyterminal stubs from the cell extracts. The material was analyzed on a Tris⋅tricine 10–20% gel. The lower part of the figure shows a stronger exposure of the bottom part of the gel to better reveal the APP carboxyterminal fragments. (B) Analysis of Aβ secretion into the medium (23, 38). The conditioned medium of the same neurons examined in A was immunoprecipitated with the Aβ antibody B7/6. This antibody recognizes an epitope between the α- and the β-secretase sites and therefore does not precipitate the p3 fragment. Material was analyzed on a Tris⋅tricine 10–20% gel.