Abstract
Recruitment of acid hydrolases to lysosomes generally occurs by intracellular sorting based on recognition of a common mannose 6-phosphate signal in the transGolgi network and selective transport to late endosomes/lysosomes. Here we provide evidence for an alternative, efficient secretion-recapture pathway mediated by megalin and exemplified by cathepsin B in kidney proximal convoluted tubules (PCT). We found that in mouse kidneys with defective megalin expression [megalin knockout (KO)] or apical PCT trafficking (ClC-5 KO), the (pro)cathepsin B mRNA level was essentially preserved, but the protein content was greatly decreased and the enzyme was excreted in the urine as mannose 6-phosphate-devoid species. In polarized PCT-derived cells, purified cathepsin B was avidly and selectively taken up at the apical membrane, and uptake was abolished by the megalin competitor, receptor-associated protein. Direct interaction of cathepsin B with megalin was demonstrated by surface plasmon resonance. Procathepsin B was detected in normal mouse serum. Purified cathepsin B injected into mice was efficiently taken up by kidneys (≈10% of injection) and targeted to lysosomes where it remained active, as shown by autoradiography and subcellular fractionation. A single cathepsin B injection into cathepsin B KO mice could reconstitute full lysosomal enzyme activity in the kidneys. These findings demonstrate a pathway whereby circulating lysosomal enzymes are continuously filtered in glomeruli, reabsorbed by megalin-mediated endocytosis, and transferred into lysosomes to exert their function, providing a major source of enzymes to PCT. These results also extend the significance of megalin in PCT and have several physiopathological and clinical implications.
Keywords: lysosomes, mannose 6-phosphate, megalin, cathepsin B
Correct assembly of lysosomes is essential as demonstrated by lysosomal storage diseases. Almost all newly synthesized lysosomal enzymes are targeted to lysosomes based on a common mannose 6-phosphate (M6P) recognition signal acquired in the Golgi. Sorting depends on binding to M6P receptors in the transGolgi network, which direct their cargo to clathrin-coated vesicles that fuse with endosomes to subsequently reach lysosomes (1, 2). By this mechanism, lysosomal enzymes are efficiently, but not fully, segregated from secretory proteins in the Golgi, resulting in secretion of 5%–20% of lysosomal enzymes (2). It has long been assumed that a large fraction of released enzymes is recaptured by M6P receptors present on the plasma membrane in various tissues. However, an alternative, M6P-independent targeting mechanism must exist. In patients suffering from inclusion-cell disease, defective phosphotransferase activity results in a lack of the terminal M6P recognition signal on acid hydrolases (3, 4). Multiple cell types in these patients lack essentially all lysosomal enzymes, but other tissues, including liver, kidney, spleen, and brain, exhibit normal lysosomal enzyme activities (4, 5, 6). M6P-independent targeting is further demonstrated by the analysis of mice deficient for M6P receptors, where enzyme levels are within normal values in the liver, kidney, and brain (5).
The present study clarifies the molecular basis of such an M6P-independent mechanism and reveals a pathway of lysosomal biogenesis in kidney proximal convoluted tubules (PCT). The well developed apical endocytic apparatus of these cells reflects an extraordinary capacity for reabsorption of macromolecules from the glomerular ultrafiltrate via the apical multiligand receptor megalin (7). Full KO of megalin (8) leads to severe low-molecular-weight (LMW) proteinuria (7). Prominent LMW proteinuria is also the constant hallmark of patients with Dent's disease, originally identified as X-linked nephrolithiasis (9). This disease is reproduced in KO mice for ClC-5 (a voltage-gated chloride/proton antiporter), where proteinuria is caused by defective trafficking of megalin to the apical plasma membrane of PCT (10). In these mice, we have earlier observed that the kidney content of N-acetyl β-hexosaminidase and cathepsin B are markedly decreased, together with abnormally high urinary excretion of these enzymes (10). Here we show that megalin-mediated endocytosis of filtrated cathepsin B is the main source for lysosomal supply of enzymatically active cathepsin B to PCT, and we propose that this pathway may be operating for other LMW soluble lysosomal constituents, providing a major contribution to the lysosomal function of PCT.
Results
Renal and Urinary Cathepsin B Protein Content Correlate with Megalin Function.
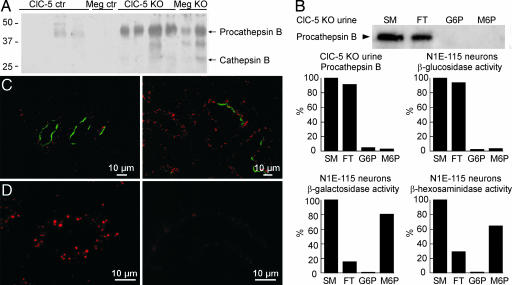
Based on our previous observations of increased urinary excretion of lysosomal enzymes in ClC-5 KO mice, we first looked for cathepsin B, a major lysosomal cysteine protease with high activity in kidney PCT (11), in the urine from full megalin KO mice. Similar to ClC-5 KO mice, urine from megalin KO mice showed a strong signal for procathepsin B (Mr ≈44 kDa) and a weaker signal for the mature enzyme (Mr ≈31 kDa) (Fig. 1A). Conversely, cathepsin B content was decreased in renal cortex from megalin KO mice (data not shown), as shown previously for ClC-5 KO mice (10). These data point to a similar defect of lysosomal biogenesis in kidney PCT of ClC-5 and megalin KO mice.
Fig. 1.
Cathepsin B protein levels in PCT and urine correlate with megalin function. (A) Immunoblot of urines from 4 ClC-5 and 2 full megalin KO mice and corresponding control mice by using rabbit anti-rat cathepsin B antibodies. (B) Affinity-chromatography on immobilized M6P receptor of procathepsin B from ClC-5 KO mice urine, compared with neuronal β-galactosidase and β-hexosaminidase (positive controls) and β-glucosidase (negative control). SM, starting material; FT, flow-through; G6P, sham-elution with glucose 6-phosphate; M6P; specific elution with M6P. (C) Immunofluorescence of kidney from kidney-specific megalin KO mice by using sheep anti-megalin (green), rabbit anti-rat cathepsin B (red, Left), and rat anti-LAMP1 antibodies (red, Right). (Left) Shows three PCT where several cells had preserved megalin expression among a majority of PCT devoid of megalin. (D) Immunofluorescence of kidney from a ClC-5 KO (Right) and a control mouse (Left) labeled with rabbit anti-rat cathepsin B antibody.
Next, we investigated the presence of M6P on procathepsin B recovered from the urine of ClC-5 KO mice to address our previous hypothesis that urinary excretion was caused by defective intracellular targeting of newly synthesized lysosomal enzymes in PCT (10). Procathepsin B from ClC-5 KO urine failed to bind to immobilized M6P receptors, in contrast to neuronal β-galactosidase and β-hexosaminidase, which retain their M6P moiety in these cells and served as positive controls (Fig. 1B). Likewise, neither procathepsin B occurring in mouse plasma nor purified human liver cathepsin B (used for studies described in Figs. 2, 3, and 4) were detectably retained by the M6P column (data not shown). These observations argued against a primary intracellular retention defect and indicated instead that cathepsin B, released into the blood by various cells in the body (2), could be filtered in the glomeruli and reabsorbed into PCT by megalin-mediated endocytosis. To test this hypothesis at the cellular level, kidney-specific megalin KO mice were analyzed by immunohistochemistry. In these animals, incomplete Cre-recombinase activity leads to mosaic PCT, with cells still expressing megalin scattered among megalin-defective ones (12). There was a perfect correlation between megalin expression and a strong procathepsin B signal in individual cells (Fig. 1C Left). Likewise, the level of cathepsin D in individual PCT cells depended on the presence of megalin (data not shown). However, megalin KO results in severe atrophy of the apical endocytic apparatus in PCT (13). Therefore, we also compared the abundance of vesicles bearing the lysosomal membrane marker LAMP-1 to exclude the possibility that the decrease in PCT content of cathepsin B and D would reflect a reduced capacity of lysosomes. No difference in LAMP-1 signal between adjacent megalin-expressing and -defective cells was noticed (Fig. 1C Right).
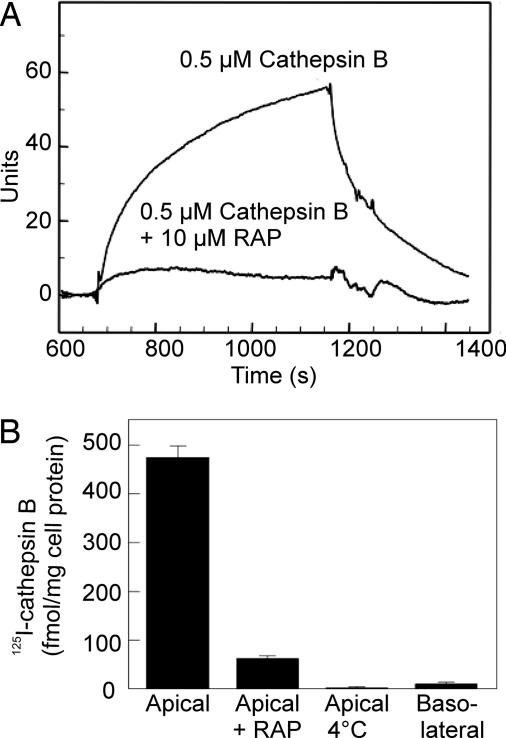
Fig. 2.
Cathepsin B uptake is mediated by megalin. (A) Biacore analysis of human cathepsin B binding to megalin and its inhibition by RAP (signal by RAP alone has been subtracted). (B) Apical endocytic uptake of 125I human cathepsin B in PCT-derived polarized OK cells and its inhibition by RAP (mean ± SEM, n = 3).
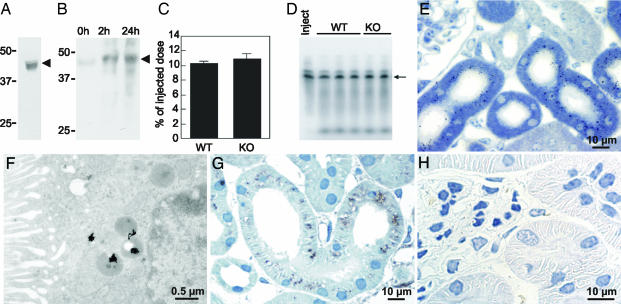
Fig. 3.
Cathepsin B is filtered from plasma and taken up in PCT. (A) Immunoblot of WT mouse serum with goat anti-mouse cathepsin B antibodies. (B) Immunoblot of WT renal cortex at 0, 2, and 24 h after injection of recombinant N-tagged procathepsin B by using an anti-tag antibody. (C) Renal uptake of injected 125I-cathepsin B in WT and cathepsin B KO mice at 1 h after injection (mean ± SEM, n = 3). (D) PhosphorImager analysis showing the integrity of injected 125I-cathepsin B in kidneys from WT and cathepsin B KO mice at 1 h after injection. Arrowheads at A and B, position of procathepsin B; arrow at D, position of mature cathepsin B. (E and F) Light (E) and electron (F) microscopic autoradiography of kidney cortex from a normal mouse at 2 h after 125I-cathepsin B injection. (G) Immunoperoxidase by using rabbit anti-rat cathepsin B antibodies on renal cortex 2 h after injection of 7 μg of human cathepsin B into cathepsin B KO mouse. (H) Immunoperoxidase by using rabbit anti-rat cathepsin B antibodies on renal cortex from an uninjected cathepsin B KO mouse.
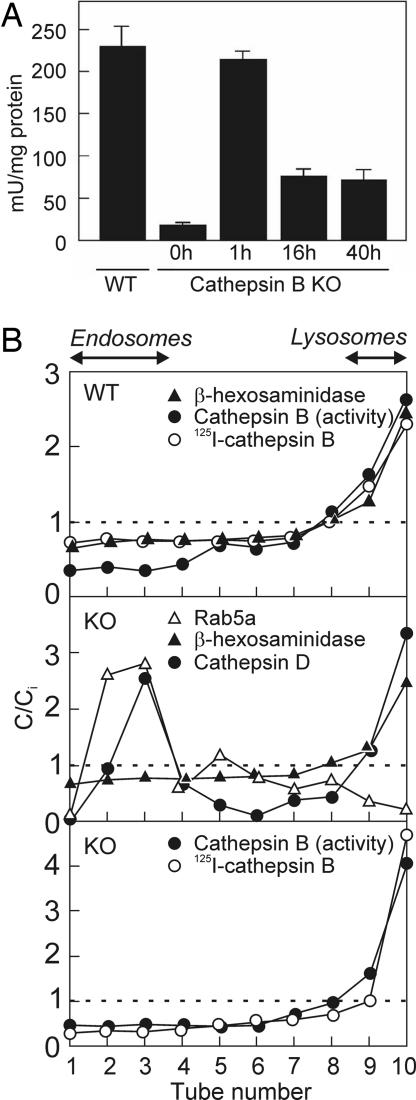
Fig. 4.
Endocytosed cathepsin B is targeted to lysosomes and remains active. (A) Enzymatic assay for cathepsin B in postnuclear particles of noninjected WT and cathepsin B KO mice, and in cathepsin B KO mice injected with 50 μg human cathepsin B at 1, 16, and 40 h after injection. Values are expressed as the activity sensitive to the specific cathepsin B inhibitor, CA-074. Background values (activity refractory to the inhibitor) were negligible in WT, but significant in KO mice, and were not subtracted (mean ± SEM, n = 3). (B) Density distributions in kidney postnuclear particles of WT (Top) and cathepsin B KO mice (Middle and Bottom) exemplified at 16 h after injection of 125I-cathepsin B (WT and KO) or 50 μg nonlabeled cathepsin B (KO). C/Ci indices >1 represent enrichment level over initial concentrations (dotted lines). Distributions of tracers are representative of at least three experiments and were comparable at 1 h after injection.
At the electron microscopic level, immunogold labeling on kidney-specific megalin KO mice confirmed the striking contrast between high levels of cathepsin B in megalin-expressing PCT cells and its almost absence in adjacent megalin-deficient cells [supporting information (SI) Fig. 5]. Colocalization of cathepsin B with LAMP-1 in megalin-expressing cells demonstrated targeting of cathepsin B to lysosomes (SI Fig. 5). In addition, comparison of immunolabeling for cathepsin B in ClC-5 KO mice and wild-type (WT) littermates showed a strong decrease in PCT cathepsin B levels upon ClC-5 gene inactivation (Fig. 1D, WT Left, KO Right). Measurement by real-time PCR of cathepsin B, cathepsin D, β-hexosaminidase, and LAMP-1 mRNA in kidney cortices from full megalin KO, kidney-specific megalin KO, and ClC-5 KO mice, as compared with respective controls, showed that expression levels of all lysosomal constituents tested were essentially unaffected by megalin and ClC-5 gene inactivation (SI Fig. 6). This result strongly argued against the possibility that decreased abundance of cathepsin B and D in PCT was due to decreased transcription.
Cathepsin B Is a Megalin Ligand.
To more directly test whether megalin could be responsible for the supply of cathepsin B and D to PCT lysosomes, we investigated whether the purified enzymes were able to interact with this receptor by using surface plasmon resonance analysis. Cathepsin B and D were found to bind megalin-coated sensor chips with an approximate Kd of 400 nM (Fig. 2A) and 1 μM, respectively (data not shown). Interaction of both enzymes was disrupted by the receptor-associated protein (RAP), a chaperone for megalin (14, 15), that prevents association of all known ligands with the receptor. The involvement of megalin in the cellular uptake of cathepsin B was confirmed by studies in polarized PCT-derived opossum kidney (OK) cells. 125I-cathepsin B was avidly internalized from the apical medium at 37°C, but not from the basolateral medium, or at 4°C, and apical uptake was blocked by coincubation with RAP (Fig. 2B).
Filtration and Proximal Tubular Uptake of Procathepsin B.
For megalin-mediated uptake of procathepsin B in PCT to be of physiological relevance, the enzyme should be found in significant amounts in serum and be filtered through renal glomeruli. Procathepsin B was readily detected in mouse serum (Fig. 3A) without detectable M6P (see above), presumably reflecting default secretion of procathepsin B from various cell types (11). Cultured fibroblasts are known to release ≈5% of newly synthesized procathepsin B (16).
To further investigate whether circulating procathepsin B could be significantly filtrated and taken up into renal PCT cells, recombinant N-tagged procathepsin B was injected into WT mice. Immunoblot analysis with an anti-tag antibody clearly identified exogenous procathepsin B in renal cortices (Fig. 3B). We next measured the efficiency of the uptake of trace amounts of 125I-human liver cathepsin B (devoid of detectable M6P) in kidneys. Uptake reached 10% of the injected dose after 1 h in both WT and cathepsin B KO mice (Fig. 3C), and the tracer remained intact as shown by PhosphorImager analysis (Fig. 3D). By autoradiography, 125I-cathepsin B was restricted to the apical cytoplasm of PCT after 2 h (Fig. 3E) and concentrated in clustered electron-dense organelles identified as lysosomes (Fig. 3F). In addition, cathepsin B antigenicity was demonstrated by immunohistochemistry in kidney PCT of injected cathepsin B KO mice (Fig. 3G), but not in noninjected littermates (Fig. 3H).
Exogenous Enzyme Can Reconstitute Full Cathepsin B Activity in Lysosomes of Cathepsin B KO Mice.
We finally investigated whether this pathway could allow exogenous enzyme to reconstitute a normal cathepsin B activity in cathepsin B KO mouse kidneys, assuming a similar 10% uptake efficiency of a high dose of unlabeled enzyme as observed for the tracer amount of 125I-cathepsin B. After injection of nonradiolabeled cathepsin B into cathepsin B KO mice at an ≈10-fold higher activity than found in WT mouse kidneys, renal cathepsin B activity was fully restored after 1 h and remained at about one-fourth the normal value after 16–40 h (Fig. 4A). When kidney postnuclear particles were equilibrated in Percoll gradients, injected cathepsin B (125I in WT and cathepsin B KO mice, unlabeled in KO) was restricted to the densest fractions, perfectly codistributing with β-hexosaminidase and endogenous cathepsin B in WT, and well resolved from endosomes (Rab5a). The bimodal distribution of cathepsin D, with a low-density endosomal peak and a high-density lysosomal peak (Fig. 4B), probably reflects its predominant expression in collecting ducts (17).
Discussion
Lysosomal enzymes are generally targeted by intracellular M6P-dependent trafficking. Our studies reveal a new pathway for lysosome biogenesis in PCT. This mechanism involves secretion of procathepsin B into plasma by cells throughout the body, filtration by renal glomeruli, megalin-mediated apical uptake in PCT, and delivery to lysosomes where the enzyme is active, thereby providing a major contribution to the lysosomal function.
This M6P-independent mechanism likely explains why inclusion-cell disease patients and M6P-receptor-deficient mice maintain normal lysosomal enzyme activities in kidneys (4, 5). Likewise, lysosomal delivery of the obligatory activators for lysosomal sphingolipid metabolism, called saposins, is supported by a megalin-related member of the low-density lipoprotein receptor family, the low-density lipoprotein-related protein, or by mannose receptors (18). Collectively, M6P-independent mechanisms could account for the limited extracellular accumulation of secreted lysosomal enzymes having lost M6P due to phosphatase activities and could contribute to the benefits of enzyme replacement therapy of lysosomal storage disorders, at least in some tissues.
Megalin-mediated uptake of cathepsin B in kidney PCT is remarkably efficient because its enzyme activity can be fully restored in kidneys of cathepsin B KO mice by a single i.v. injection, with a significant activity remaining for up to 40 h. However, under normal conditions, glomerular filtration of cathepsin B and presumably other LMW soluble lysosomal constituents is continuous, thereby constantly feeding PCT cells with fresh enzymes. The easy detection of cathepsin B in normal serum and in urines from megalin and ClC-5 KO mice, where glomerular filtration is normal but reuptake is strongly impaired, suggests that permanent filtration of the enzymes provides a major supply for the PCT. From a physiological perspective, a mechanism whereby proteases are filtered and internalized together with their substrates would enhance proteolytic supply to lysosomes when demands increases, thereby spontaneously conferring adaptable protection against toxicity of protein overload. From a pathological perspective however, because megalin is a multiligand scavenger, the lysosomal supply of cathepsin B and other acid hydrolases via this pathway might be compromised by competition upon severe glomerular proteinuria, which could play a role in tubular epithelial cell damage and progressive interstitial disease secondary to glomerular injury (19). Such competition would readily explain the increased cathepsin B activity observed in the urine upon proteinuric conditions such as type 2 diabetes mellitus (20), which so far has been attributed to increased PCT excretion of the enzyme. Our results show instead, in two models of tubular proteinuria, that a high urinary level of cathepsin B is caused by impaired reabsorption, not by elevated secretion. This conclusion can be drawn from three main observations. First, megalin and ClC-5 KO mice have no glomerular dysfunction, but do have defective receptor-mediated uptake of filtered proteins. Second, megalin acts as a receptor for cathepsin B, as observed by RAP inhibitable uptake in OK cells and by surface plasmon analysis. Third, urinary cathepsin B contains no detectable M6P, arguing against a primary defect of intracellular sorting. Accordingly, we propose that elevated urinary levels of cathepsin B in diabetes and other glomerular diseases result from the combination of increased filtration with decreased megalin-mediated reuptake due to competition from increased protein load.
The urinary content of another lysosomal enzyme, N-acetyl-β-glucosaminidase, also known as β-hexosaminidase (130 kDa), is routinely used as a clinical marker for early detection of renal tubular impairment in glomerular disease (21) and is generally regarded as reflecting release from PTC. We propose to reassess the significance and usefulness of this assay. Although glomerular filtration of a 130-kDa-large protein is obviously questionable, transferrin (80 kDa) and even bile salt-dependent lipase (110 kDa) are filtered under normal conditions (22, 23), and increased glomerular leakage will increase filtered levels. Because cathepsin B has a much lower molecular weight, and consequently a much higher filtration coefficient than N-acetyl-β-glucosaminidase, we suggest that cathepsin B could be an alternative marker for tubular dysfunction in glomerular disease.
In conclusion, the present work establishes the major contribution of apical receptor-mediated endocytosis to lysosomal biogenesis in the kidney PCT. It further emphasizes the broad role of megalin in this epithelium, extending its function from reabsorption and maintenance of homeostasis of a variety of molecules to enzyme recruitment.
Materials and Methods
Antibodies and Tracers.
As antibodies, we used rabbit anti-rat and goat anti-mouse cathepsin B (Upstate Biotechnology, Lake Placid, NY; R&D Systems, Minneapolis, MN), rabbit anti-Rab5a, goat anti-cathepsin D (Santa Cruz Biotechnology, Santa Cruz, CA; Upstate Biotechnology), sheep anti-megalin (kindly provided by P. J. Verroust, INSERM U538, Paris, France), rat anti-LAMP-1 (Developmental Studies Hybridoma Bank, University of Iowa), and anti-Xpress-HRP (Invitrogen, Carlsbad, CA). Purified human liver cathepsin B was from Calbiochem (San Diego, CA). Procathepsin B tagged in the N terminus with an Xpress epitope was synthesized by the Expressway Plus Expression system (Invitrogen) after cloning procathepsin B into pEXP1-DEST (Invitrogen).
Animals and Urine Samples.
KO mice for ClC-5, megalin (full and kidney-specific), and cathepsin B were generated by gene targeting in a C57 black background as described (8, 12, 24, 25). Gender- and age-matched WT littermates were used as controls. Kidneys were flushed by perfusion through the abdominal aorta before homogenization or perfusion fixation. Urine was collected from the bladder before perfusion and immediately frozen. Studies were carried out in accordance with regulations of the National Institutes of Health for the care and use of laboratory animals.
Immunoblotting.
After perfusion with saline, kidney cortices were homogenized in ice-cold 0.3 M sucrose, 25 mM imidazole (pH 7.2), with 8.5 μM leupeptin, 1 mM PMSF, and 1 mM EDTA for 30 sec with an IKA Ultra-turrax T8 homogenizer (IKA 0Werke, Staufen, Germany) and centrifuged at 4,000 × g for 15 min at 4°C. Cleared supernatants and urines were analyzed by SDS/PAGE as described (26).
Affinity-Chromatography on Immobilized M6P Receptor.
Affinity-chromatography of urine samples from 3 ClC-5 KO mice was performed on the soluble fragment of the cation-independent M6P receptor immobilized on Affigel column at 4°C in PBS/1% Triton X-100 in the presence of complete protease inhibitors (Roche Diagnostics, Indianapolis, IN) as described (27). Samples were diluted in 1 ml and allowed to bind for 10 min. After nonbound material was collected as flow-through and extensive washing, the column was sequentially eluted with glucose 6-phosphate and M6P (10 mM each, Sigma–Aldrich, St. Louis, MO). Because some brain lysosomal enzymes retain M6P (27), the column was further tested by using extracts of NIE-115 neurons, with assays of β-galactosidase and β-hexosaminidase as positive controls and β-glucosidase, which is targeted to lysosomes by a M6P-independent mechanism, as a negative control.
Immunohistochemistry and Cytochemistry.
Tissues were processed for light and electron microscopy as described (28). For immunolocalization of exogenous cathepsin B, cathepsin B KO mice were injected into the femoral vein with 7 μg human liver cathepsin B in 100 μl. Immunoperoxidase and immunofluorescence images were acquired with a Leica DMR microscope equipped with a Leica DFC320 camera (Wetzlar, Germany). Images were transferred by a Leica TFC Twain 6.1.0 program and processed by using Adobe Photoshop 8.0 (Adobe Systems, Mountain View, CA). Electron micrographs were obtained by using a Phillips CM 100 electron microscope and digitally transferred by AnalySIS.
Real-Time PCR.
Real-time PCR was performed as described previously (29, 30). The mRNA levels of target genes were adjusted to GAPDH mRNA level, and relative changes in mRNA levels in megalin and ClC-5 KO mice were determined by comparison to the WT mRNA level in corresponding littermates by using the following formula: ratio = (Etarget)Δct (WT-KO)/(EGAPDH)Δct (WT-KO) (29, 30). The primers (SI Table 1) were designed by using Beacon Designer 2.0 (Premier Biosoft, International, CA).
Surface Plasmon Resonance Analysis.
Biacore sensor chips (type CM5; Biacore, Uppsala, Sweden) were activated with a 1:1 mixture of 0.2 M N-ethyl-N′-(3-dimethylaminopropyl) carbodiimide and 0.05 M N-hydroxysuccinimide in water according to the manufacturer's recommendations. Rat megalin, purified by RAP affinity-chromatography, was immobilized as described (31). Flow cells were regenerated with 1.5 M glycine/HCl (pH 3.0), and flow buffer was 150 mM NaCl, 10 mM Hepes (pH 7.4), with 1.5 mM CaCl2 and 1 mM EGTA. Binding data were analyzed by using the BIA evaluation program.
Uptake of 125I-Cathepsin B.
Human liver cathepsin B (Calbiochem) was radioiodinated in iodogen-coated tubes (Pierce, Rockford, IL) to ≈30,000 cpm/ng. In vivo kidney uptake was measured at 1 h after i.v. injection of 15 × 106 cpm 125I-cathepsin B in 100 μl of saline into WT and cathepsin B KO mice. Integrity was tested by PhosphorImager analysis of postnuclear particles by SDS/PAGE gels (15% acrylamide) after full separation (Fig. 3D) or 1/3 migration to retain free radioiodine in the wet gel (not detectable in kidney fractions). KO cells were plated at 2.15 × 105 cells/cm2 on Transwell filters (Costar, Cambridge, MA) and used after 4 days, at which time transepithelial resistance had reached ≈300 Ohm cm2. 125I-cathepsin B (1.8 nM) was taken up from the apical or basolateral medium for 1 h at 37°C or 2 h at 4°C in the absence or presence of 180 nM recombinant RAP (a kind gift of Dr. M. S. Nielsen, Aarhus University, Denmark).
Autoradiography.
Cathepsin B was localized by autoradiography in kidney cortex sections after injection into the femoral vein with 55 × 106 cpm 125I-cathepsin B in 100 μl of saline. For light and electron microscopic autoradiography, kidneys were fixed by perfusion with 1% glutaraldehyde in 0.1 M sodium cacodylate buffer, followed by postfixation, dehydration, and embedding into Epon 812, and then sections were processed for autoradiography by using Amersham LM-1 or Ilford L4 emulsion, respectively (Amersham Biosciences, Piscataway, NJ; Ilford, Cheshire, U.K.).
Analytical Subcellular Fractionation and Assays of Cathepsin B Activity.
Kidney homogenates were analyzed by differential sedimentation followed by density centrifugation of postnuclear particles in 16% Percoll as described (11). Fractions were analyzed for the activity of N-acetyl-β-hexosaminidase and cathepsin B (endogenous in WT, injected in KO), 125I cathepsin B counts, as well as Rab5a and mature cathepsin D protein levels (10). Cathepsin B activity was measured at pH 6.0 by the coumarin-based assay (32) (postnuclear particles) or the naphtylamine-based assay (33) (Percoll gradients) as the difference between values in the absence or presence of specific inhibition by 100 μM CA-074 (Sigma–Aldrich). Cathepsin B activity was completely inhibited in WT mice; the significant residual value in cathepsin B KO mice indicates up-regulation of other acid protease(s) incompletely blocked by this inhibitor (34) (the low residual signal in noninjected KO mice was not subtracted from injected KO mice).
Supplementary Material
Acknowledgments
We thank Dr. T. Reinheckel (Freiburg, Germany) for generously providing cathepsin B KO mice, Dr. P. Lobel (Rutgers University, Piscataway, NJ) for the M6P affinity matrix, Dr. M. S. Nielsen (Aarhus, Denmark) for the gift of RAP, and Dr. P. J. Verroust (Paris, France) for megalin antibodies. Assistance by H. Debaix, A. M. Haas, I. B. Kristoffersen, M. Leruth, Y. Marchand, P. Nielsen, F. N. Kuli, and H. Sidelmann is greatly acknowledged. This work was supported by Fonds National de la Recherche Scientifique, InterUniversity Attraction Poles, King Baudouin Foundation, Concerted Research Actions, Région Wallonne, Danish Medical Research Council, University of Aarhus, NOVO-Nordisk Foundation, Biomembrane Research Center, and European Commission (EU Framework Progam 6, EureGene).
Abbreviations
- ClC-5
voltage-gated proton/chloride antiporter
- KO
knockout
- LMW
low molecular weight
- M6P
mannose 6-phosphate
- OK
opossum kidney
- PCT
proximal convoluted tubules
- RAP
receptor-associated protein
- WT
wild type.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700330104/DC1.
References
- 1.Natowicz M-R, Chi M-M, Lowry O-H, Sly W-S. Proc Natl Acad Sci USA. 1979;76:4322–4326. doi: 10.1073/pnas.76.9.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kornfeld S. J Clin Invest. 1986;77:1–6. doi: 10.1172/JCI112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reitman M-L, Varki A, Kornfeld S. J Clin Invest. 1981;67:1574–1579. doi: 10.1172/JCI110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waheed A, Pohlmann R, Hasilik A, von Figura K, van Elsen A, Leroy J-G. Biochem Biophys Res Commun. 1982;105:1052–1058. doi: 10.1016/0006-291x(82)91076-2. [DOI] [PubMed] [Google Scholar]
- 5.Dittmer F, Ulbrich E-J, Hafner A, Schmahl W, Meister T, Pohlmann R, von Figura K. J Cell Sci. 1999;112:1591–1597. doi: 10.1242/jcs.112.10.1591. [DOI] [PubMed] [Google Scholar]
- 6.Kornfeld S, Sly W-S. In: The Metabolic and Molecular Bases of Inherited Diseases. Scriver C-R, Beaudet A-L, Sly W-S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 2495–2508. [Google Scholar]
- 7.Christensen E-I, Birn H. Nat Rev Mol Cell Biol. 2002;3:256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- 8.Willnow T-E, Hilpert J, Armstrong S-A, Rohlmann A, Hammer R-E, Burns D-K, Herz J. Proc Natl Acad Sci USA. 1996;93:8460–8464. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd S-E, Pearce S-H, Fisher S-E, Steinmeyer K, Schwappach B, Scheinman S-J, Harding B, Bolino A, Devoto M, Goodyer P, et al. Nature. 1996;379:445–449. doi: 10.1038/379445a0. [DOI] [PubMed] [Google Scholar]
- 10.Christensen E-I, Devuyst O, Dom G, Nielsen R, van der Smissen P, Verroust P, Leruth M, Guggino W-B, Courtoy P-J. Proc Natl Acad Sci USA. 2003;100:8472–8477. doi: 10.1073/pnas.1432873100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mort J-S. In: Handbook of Proteolytic Enzymes. Barrett A-J, Rawlings N-D, Woessner FJ, editors. San Diego: Academic; 2004. pp. 1079–1086. [Google Scholar]
- 12.Leheste J-R, Melsen F, Wellner M, Jansen P, Schlichting U, Renner-Muller I, Andreassen T-T, Wolf E, Bachmann S, Nykjaer A, et al. FASEB J. 2003;17:247–249. doi: 10.1096/fj.02-0578fje. [DOI] [PubMed] [Google Scholar]
- 13.Christensen E-I, Willnow T-E. J Am Soc Nephrol. 1999;10:2224–2236. doi: 10.1681/ASN.V10102224. [DOI] [PubMed] [Google Scholar]
- 14.Willnow T-E, Rohlmann A, Horton J, Otani H, Braun J-R, Hammer R-E, Herz J. EMBO J. 1996;15:2632–2639. [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen E-I, Gliemann J, Moestrup S-K. J Histochem Cytochem. 1992;40:1481–1490. doi: 10.1177/40.10.1382088. [DOI] [PubMed] [Google Scholar]
- 16.Achkar C, Gong Q-M, Frankfater A, Bajkowski A-S. J Biol Chem. 1990;265:13650–13654. [PubMed] [Google Scholar]
- 17.Yokota S, Tsuji H, Kato K. J Histochem Cytochem. 1985;33:191–200. doi: 10.1177/33.3.2579120. [DOI] [PubMed] [Google Scholar]
- 18.Hiesberger T, Huttler S, Rohlmann A, Schneider W, Sandhoff K, Herz J. EMBO J. 1998;17:4617–4625. doi: 10.1093/emboj/17.16.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nangaku M, Shankland S-J, Couser W-G. J Am Soc Nephrol. 2005;16:1195–1204. doi: 10.1681/ASN.2004121098. [DOI] [PubMed] [Google Scholar]
- 20.Piwowar A, Knapik-Kordecka M, Fus I, Warwas M. Med Sci Monit. 2006;12:CR210–CR214. [PubMed] [Google Scholar]
- 21.Skalova S. Acta Medica (Hradec Kralove) 2005;48:75–80. [PubMed] [Google Scholar]
- 22.Kozyraki R, Fyfe J, Verroust P-J, Jacobsen C, Dautry-Varsat A, Gburek J, Willnow T-E, Christensen E-I, Moestrup S-K. Proc Natl Acad Sci USA. 2001;98:12491–12496. doi: 10.1073/pnas.211291398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comte B, Franceschi C, Sadoulet M-O, Silvy F, Lafitte D, Benkoel L, Nganga A, Daniel L, Bernard J-P, Lombardo D, et al. Kidney Int. 2006;69:1048–1055. doi: 10.1038/sj.ki.5000133. [DOI] [PubMed] [Google Scholar]
- 24.Wang S-S, Devuyst O, Courtoy P-J, Wang X-T, Wang H, Wang Y, Thakker R-V, Guggino S, Guggino W-B. Hum Mol Genet. 2000;9:2937–2945. doi: 10.1093/hmg/9.20.2937. [DOI] [PubMed] [Google Scholar]
- 25.Deussing J, Roth W, Saftig P, Peters C, Ploegh H-L, Villadangos J-A. Proc Natl Acad Sci USA. 1998;95:4516–4521. doi: 10.1073/pnas.95.8.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai J, Christensen E-I, Morris S-M, Willnow T-E, Cooper J-A, Nielsen R. Am J Physiol Renal Physiol. 2005;289:F569–F576. doi: 10.1152/ajprenal.00292.2004. [DOI] [PubMed] [Google Scholar]
- 27.Sleat D-E, Sohar I, Lackland H, Majercak J, Lobel P. J Biol Chem. 1996;271:19191–19198. doi: 10.1074/jbc.271.32.19191. [DOI] [PubMed] [Google Scholar]
- 28.Christensen E-I, Nielsen S, Moestrup S-K, Borre C, Maunsbach A-B, de Heer E, Ronco P, Hammond T-G, Verroust P. Eur J Cell Biol. 1995;66:349–364. [PubMed] [Google Scholar]
- 29.Jouret F, Igarashi T, Gofflot F, Wilson P-D, Karet F-E, Thakker R-V, Devuyst O. Kidney Int. 2004;65:198–208. doi: 10.1111/j.1523-1755.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl M-W. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moestrup S-K, Birn H, Fischer P-B, Petersen C-M, Verroust P-J, Sim R-B, Christensen E-I, Nexø E. Proc Natl Acad Sci USA. 1996;93:8612–8617. doi: 10.1073/pnas.93.16.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo M, Mathieu P-A, Linebaugh B, Sloane B-F, Reiners J-J., Jr J Biol Chem. 2002;277:14829–14837. doi: 10.1074/jbc.M108180200. [DOI] [PubMed] [Google Scholar]
- 33.Barrett A-J. Anal Biochem. 1976;76:374–376. doi: 10.1016/0003-2697(76)90298-0. [DOI] [PubMed] [Google Scholar]
- 34.Friedrichs B, Tepel C, Reinheckel T, Deussing J, von Figura K, Herzog V, Peters C, Saftig P, Brix K. J Clin Invest. 2003;111:1733–1745. doi: 10.1172/JCI15990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.