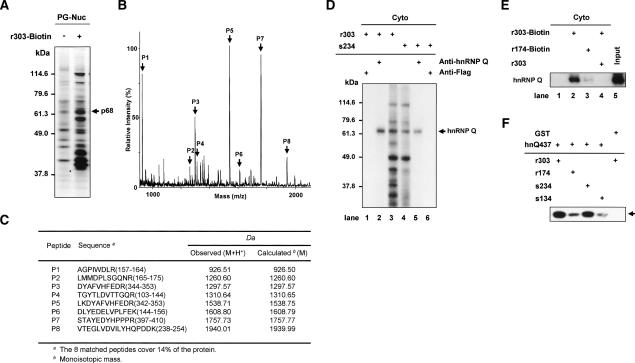
Figure 4.
Identification of p68 as hnRNP Q. (A–C) Nuclear extracts from pinealocytes (PG-Nuc) were incubated with or without a biotinylated probe (r303-Biotin), and then the resin-bound proteins were fractionated by SDS-PAGE, and silver stained in A. Peptide mass fingerprinting via MALDI-TOF was performed. (B) The peaks labeled with arrows match the estimated tryptic peptide masses of hnRNP Q with an error of <50 ppm. (C) Oligopeptide sequences from mass fingerprinting analysis. (D) Cytoplasmic extracts labeled by UV cross-linking with radiolabeled AANAT 5′UTRs were subjected to immunoprecipitation with antibodies against hnRNP Q or Flag as a control, separated by SDS-PAGE, and autoradiographed. (E) RNA pull-down experiments were performed with cytoplasmic extracts of rat pineal glands and biotinylated RNA (r303-Biotin and r174-Biotin) in the presence or absence of competitor r303. Purified proteins were immunoblotted using anti-hnRNP Q antibody. Cytoplasmic extract (Cyto) was loaded onto the “Input” lane. (F) The radiolabeled riboprobes (r303, r174, s234, and s134) were incubated with purified hnRNP Q (hnQ437) or GST, subjected to UV cross-linking analysis, and autoradiographed.