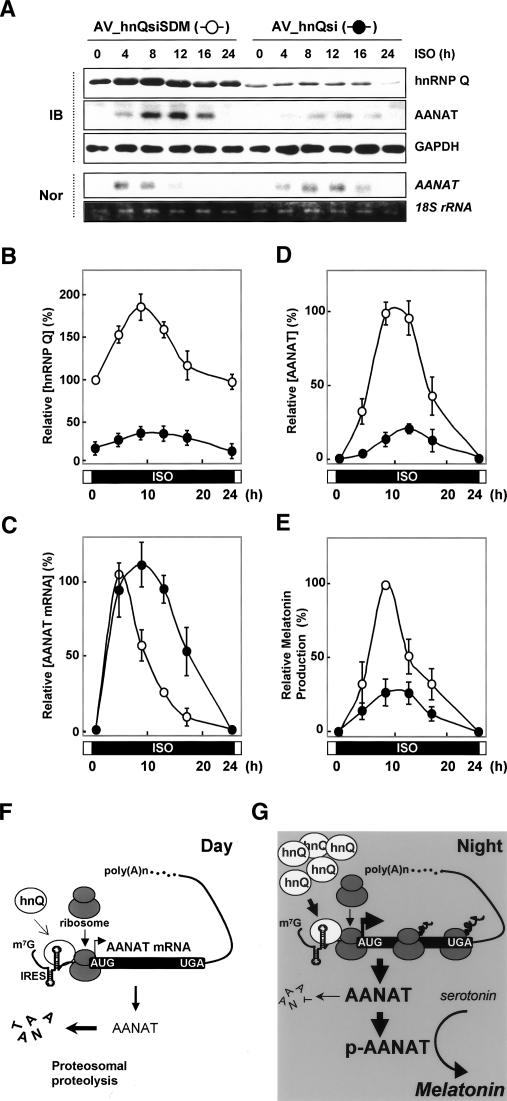
Figure 7.
Essential role of rhythmic AANAT translation in robust melatonin production. (A–D) Pinealocytes were transduced with adenoviral particles expressing either siRNA against hnRNP Q (AV_hnQsi, closed circle) or mutated siRNA sequence (AV_hnQsiSDM, open cirlce), incubated for 48 h, and treated with ISO before harvesting at the indicated times. (A) Cell lysates were extracted and subjected to immunoblotting (IB) and Northern blotting (Nor). The intensities of proteins (hnRNP Q in B and AANAT in D) and AANAT mRNA in C were quantitated with a densitometer, normalized to the GAPDH and 18S rRNA signals, respectively, and plotted as a percentage. The maximum value in the AV_hnQsiSDM-transduced pinealocytes was set to 100%. (E) Corresponding melatonin values are expressed as a percentage. The maximum value in the AV_hnQsiSDM-transduced pinealocytes was set to 100%. The values represent the mean ± SD of four independent experiments. (F,G) Proposed model for rhythmic AANAT translation as a key regulatory mechanism in nocturnal melatonin production. The circadian timing of AANAT protein expression can be explained by two cooperative nocturnal processes: (1) IRES-mediated AANAT synthesis up-regulated by rhythmic hnRNP Q, which peaks in the middle of the night; and (2) post-translational AANAT phosphorylation mediated by β-adrenergic signaling, which is required for its stability and enzymatic activity in melatonin biosynthesis. (hnQ) hnRNP Q; (p-AANAT) phosphorylated AANAT.