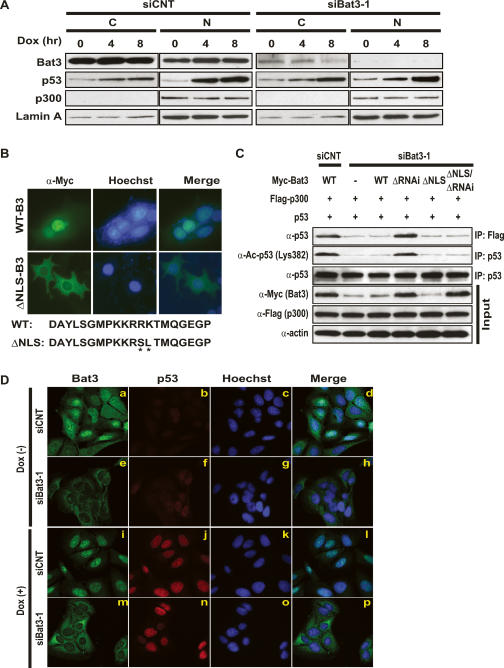
Figure 5.
Nuclear localization of Bat3 is necessary for its activity. (A) Biochemical demonstration. U2OS cells transfected with control or Bat3-specific siRNA oligo-duplexes were subjected to Dox (0.4 μg/mL) treatment. After 0, 4, or 8 h, cytosolic (C) and nuclear (N) fractions were prepared from extracts. Protein levels of Bat3, p53, and p300 were determined by immunoblotting. (Lamin A) Loading control for nuclear samples. (B) Establishment of a nuclear-localization-defective mutant of Bat3. U2OS cells transfected with Myc-tagged WT-B3 and ΔNLS-B3 were stained with anti-myc-FITC antibody. Nuclei were visualized using Hoechst 33258 (blue), merge of myc and Hoechst 33258 staining. The point mutations introduced in ΔNLS-B3 are indicated by asterisk. (C) Failure of the enhancement of p53–p300 binding and p53 acetylation by ΔNLS-B3. H1299 cells were transfected with control or Bat3-specific siRNA oligo-duplexes and cotransfected 24 h later with WT-B3, ΔRNAi-B3, ΔNLS-B3, or ΔNLS/ΔRNAi-B3. At 48 h post-transfection, p53–p300 binding and p53 acetylation were examined as in Figure 3. (D) Cellular demonstration. U2OS cells were transfected as in A and treated with Dox for 0 or 8 h, at which times the cells were fixed, permeabilized, and stained with anti-Bat3 (panels a,e,i,m) or anti-p53 (DO-1; panels b,f,j,n) antibodies, followed by anti-rabbit IgG (green) or anti-mouse IgG (red). Nuclei were visualized using Hoechst 33258 (blue; panels c,g,k,o). (Panels d,h,l,p) Merge of Bat3 and Hoechst 33258 staining. Data shown are representative of four independent preparations.