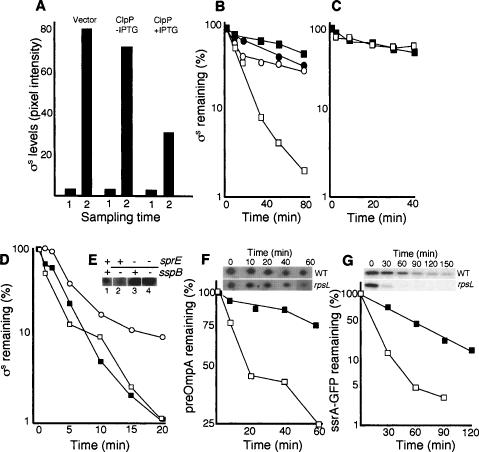
Figure 5.
Effect of overproducing ClpP and a ClpPX substrate on σS accumulation and stability. (A) Levels of σS in the cells carrying the vector control (ÅF113), cells (ÅF125) carrying the uninduced (no IPTG) Plac–clpP construct, and cells (ÅF125) induced (+IPTG) for clpP overexpression, as indicated. Sampling times are in exponential growth (1) and glucose starvation depicting the highest σS levels reached (2). (B) Stability of σS in glucose-starved wild-type cells (DV206; closed squares), cells carrying the vector control (ÅF113; closed circles), cells carrying the uninduced (no IPTG) Plac–clpP construct (ÅF125; open circles), and cells induced (+IPTG) for clpP overexpression (ÅF125; open squares). Protein synthesis was inhibited by the addition of spectinomycin after 1 h of glucose starvation. (C) Stability of σS in sprE∷tet mutant cells carrying the uninduced (no IPTG) Plac–clpP construct (ÅF135; open squares), and cells induced (+IPTG) for clpP overexpression (closed squares). Protein synthesis was inhibited by the addition of spectinomycin after 1 h of glucose starvation. (D) Stability of σS in exponentially growing wild-type cells (closed squares), cells carrying the uninduced (no IPTG) Plac–ssrA-gfp construct (ÅF142; open squares), and cells induced (+IPTG) for ssrA-gfp overexpression (open circles). (E) A knockout mutation of sspB lowers σS levels in a SprE-dependent manner. Samples were taken in the exponential growth phase (LB). The presence (+) and absence (−) of the sprE and sspB genes are indicated on top of the Western blot. The strains used are CNP119 (lane 1), CNP217 (lane 2), CNP153 (lane 3), and CNP218 (lane 4). All protein levels were determined by Western blot analysis and quantified using Image Gauge software. (F) Stability of the preOmpA in wild-type (DV206; closed squares) and rpsL141 mutant cells (ÅF1; open squares) during carbon starvation (1 h). Inset shows the preOmpA spots on two-dimensional gels after inhibition of protein synthesis in the wild type and the rpsL141 mutant. (G) Stability of the ssrA-GFP fusion protein in wild type (ÅF142; closed squares) and the rpsL141 mutant (ÅF143; open squares) during carbon starvation (1 h). Inset shows the ssrA-GFP protein on Western blots in the wild type and rpsL141 mutant after inhibition of protein synthesis. All experiments were repeated at least three times. Representative results are shown.