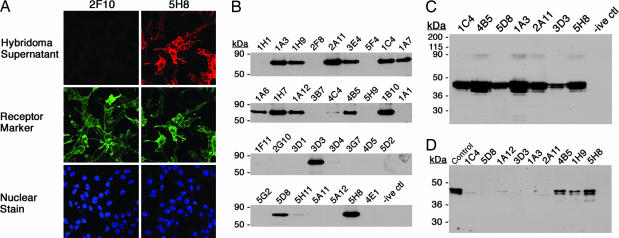
Fig. 1.
Screening of hybridoma fusions. (A) Screening by immunocytochemistry on NIH 3T3 cells expressing 5HT2c-KT3. Staining patterns from a reactive (5H8) (Top Right) and a nonreactive (2F10) (Top Left) supernatant are shown by labeling the cells, after treatment with the hybridoma supernatants, with CY3-conjugated anti-mouse IgG antibody. (Middle) Simultaneously, expression of 5HT2c was also monitored with rabbit anti-KT3 antibody on the same cells (anti-KT3 Ab) detected with FITC-conjugated anti-rabbit IgG antibody. (Bottom) In addition, in this experiment, cells were stained with nuclear stain TOTO-3. (B) Selection by IP on MBP-5HT2c expressed in E. coli. Supernatants from hybridomas for which specific staining was detected were tested for high affinity to the antigen by IP. The antigen was presented as a detergent solubilized fusion to MBP. The elutions from the IP were run on an SDS/PAGE gel and then transferred to a membrane and probed with rabbit anti-5HT2c Ab (30). The three character codes, shown on top of each lane, refer to the name assigned to that particular hybridoma. Negative control (-ive ctl) was an IP set up with growth medium for the hybridoma cells alone. (C) IP on 5HT2c expressed in mammalian cells. HEK 293 GntI− cells expressing 5HT2c were induced for the expression of this protein. The antigen was presented as a detergent-solubilized membrane fraction. The experiment was performed as discussed in B, and the blot was probed with rabbit anti-5HT2c Ab. (D) mAbs in Western blot analysis. Shown is a screen for conformationally sensitive Abs. Membranes from 293 GntI− cells expressing 5HT2c were solubilized in SDS buffer and loaded (10 μg of protein per lane) for PAGE. The membrane corresponding to each lane was cut and blotted separately with each of the mAbs. The hybridoma supernatants were diluted at 1:2,000, whereas the rabbit anti-2c control Ab was diluted at 1:10,000.