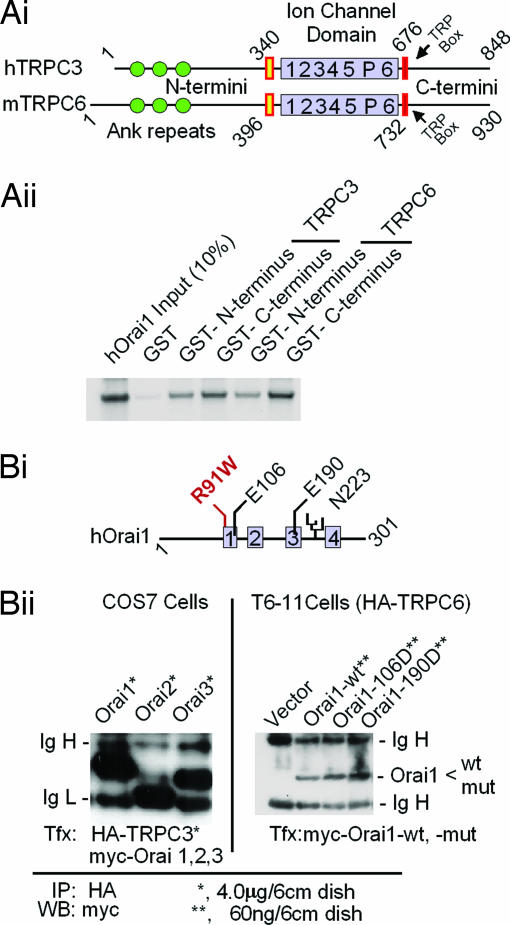
Fig. 4.
TRPC3 and TRPC6 interact with Orai in vitro (A) and in vivo (B). (Ai) Domain structures of TRPC3 and TRPC6 as deduced from their respective cDNAs. (Aii) In vitro interaction of in vitro-translated 35S-labeled N an C termini of TRPC3 and TRPC6 with Orai1 fused to GST using as binding/washing buffer 20 mM Tris·HCl (pH 7.4)/150 mM NaCl/1 mM EDTA/5 mM MgCl2/0.5% Nonidet P-40/complete protease inhibitor mixture tablets (one tablet per 50 ml). The retention of Orai1 on the fusion proteins is shown. Orai did not bind to GST without fused TRPC fragments (lane 2). (Bi) Domain structure of Orai1, location of a glycosylation site (N223), and location of mutations tested for activity. Gray boxes, likely transmembrane domains; R91W, a mutation that causes loss of SOCE in human T cells (34). (Bii Left) myc-tagged Orai proteins coimmunoprecipitate with HA-tagged TRPC3 when coexpressed in COS cells. (Bii Right) myc-tagged wild-type or mutated Orai1 coimmunoprecipitates with HA-tagged TRPC6 from T6-11 cells in which they are expressed at levels leading to functional changes. Cells were plated in 6-cm dishes and transfected with the indicated amounts of expression plasmids as for Fura-2 Ca2+ monitoring experiments (Fig. 2) (12). After 36 h at 37°C, the cells were rinsed and solubilized with RIPA buffer (50 mM Tris·HCl, pH 7.4/150 mM NaCl/1% Nonidet P-40/0.25% deoxycholate) supplemented with complete mixture of protease inhibitor tablets (one tablet per 50 ml). The lysates were cleared by centrifugation and incubated with anti-HA antibody coupled to agarose beads for 10 h in the cold. The beads were washed two times with RIPA buffer and analyzed for retention of myc-positive Orai1 by Western blotting.