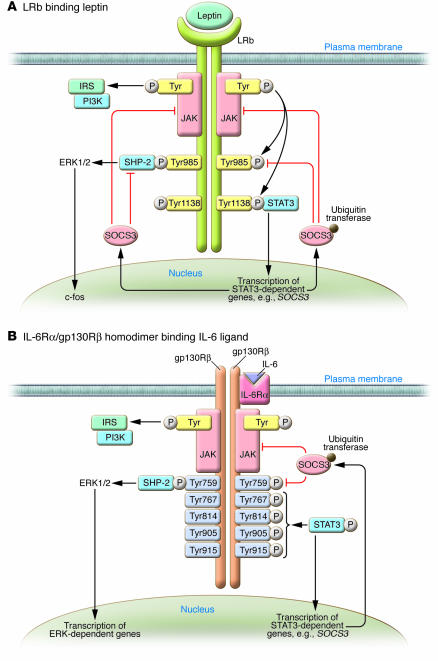
Figure 1. Signaling via LRb and gp130R: similarities and differences.
(A) Leptin binds its homodimeric receptor LRb, which results in autophosphorylation and activation of JAK, subsequently activating the insulin receptor substrate/PI3K (IRS/PI3K) signalling pathway. JAK activation also results in the phosphorylation of LRb at Tyr985 and Tyr1138. The phosphorylation of Tyr1138 mediates the recruitment, phosphorylation, and activation of the transcription factor STAT3, resulting in the transcription of SOCS3 and other STAT3-dependent genes in the nucleus. SOCS3 inhibits leptin signaling via binding to SHP-2 bound to the LRb, recruiting ubiquitin transferases to the SOCS3 box domain at Tyr985; and binding JAK. (B) Signaling through gp130Rβ is similar to that through LRb. In the case of IL-6 signaling, IL-6 binds to the IL-6Rα/gp130Rβ homodimer, which results in JAK/STAT, insulin receptor substrate/PI3K, and ERK signaling. Importantly, however, there are 4 tyrosine phosphorylation sites (at residues 767, 814, 905, and 915) distal to the SHP-2 domain bound at Tyr759. As with the LRb, SOCS3 can inhibit JAK signaling on gp130Rβ. It is not clear why gp130R ligands may overcome SOCS3 inhibition, but it may be due to the 4 additional STAT3 binding sites, since truncation of gp130R to remove these sites does not allow ligand-mediated STAT3 phosphorylation in mice in vivo (18).