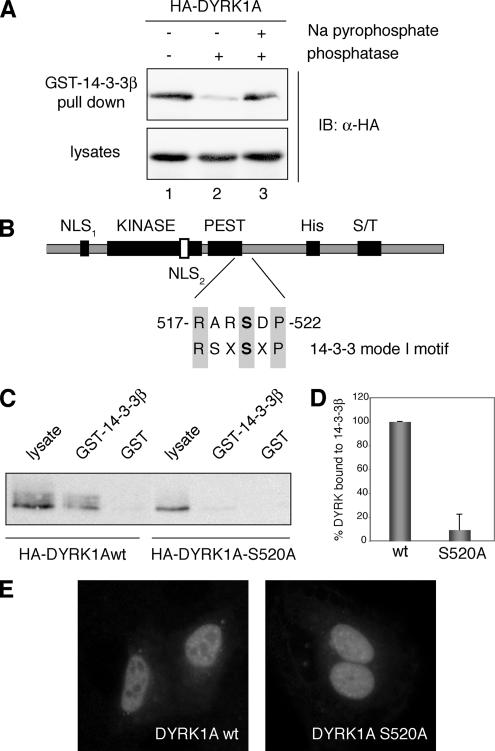
Figure 1.
Phosphorylation of Ser-520 on DYRK1A mediates its interaction with the protein 14-3-3β. (A) Lysates from HEK293 cells transiently transfected with pHA-DYRK1A were incubated for 30 min at 30°C in phosphatase buffer (lane 1) supplemented with alkaline phosphatase (lane 2) or with alkaline phosphatase and sodium pyrophosphate (lane 3) as indicated. After incubation with GST-14-3-3β immobilized on glutathione-Sepharose beads, bound protein was detected by immunoblotting (IB) with anti-HA antibody (top). An immunoblot of cell lysates representing 10% of the inputs is shown (bottom). (B) Schematic diagram of DYRK1A showing the domain structure: KINASE, kinase catalytic domain; PEST, PEST domain; His, histidine-repeat; and S/T, Ser/Thr-rich region. The sequence and the position of the putative 14-3-3 binding motif in DYRK1A are indicated and compared with a mode I 14-3-3 binding consensus sequence. The putative phosphorylated Ser residue is marked in bold. (C) HEK293 cells were transfected with pHA-DYRK1Awt and pHA-DYRK1A/S520A, and soluble lysates were incubated with unfused GST or GST-14-3-3β. Samples were analyzed by SDS-PAGE (10%) followed by immunoblotting with anti-HA antibody. Lysate represents 10% of the input. (D) The histogram presents data from three independent experiments in which the ratio bound/input in DYRK1Awt was arbitrarily set as 100. Data are expressed as a percentage of the mean value. Error bars represent SEM (standard error of the mean). (E) Subcellular localization of HA-DYRK1Awt and the mutant HA-DYRK1A/S520A, expressed in U2-OS cells, by indirect immunofluorescence analysis with anti-HA antibody followed by a FITC-conjugated anti-mouse secondary antibody.