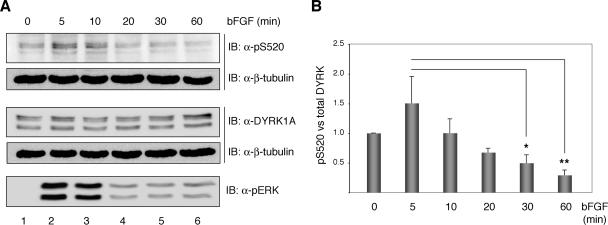
Figure 3.
bFGF modulate the phosphorylation status of DYRK1A Ser-520 in PC12 cells. (A) PC12 cells were serum-deprived for 20 h and then left unstimulated or stimulated with 100 ng/ml bFGF for the times indicated. Total extracts were resolved by 8% SDS-PAGE and analyzed by immunoblot on two independent membranes for the presence of phosphorylated Ser-520 and total DYRK1A. Both membranes were reprobed with anti β-tubulin antibody to confirm equal loading. Phosphorylation of the MAPK extracellular signal-regulated kinase (ERK) was detected by immunoblot with anti-phospho-p44/42 ERK (Thr202/Tyr204) antibody. (B) The chart shows the quantification of the phosphorylation levels of DYRK1A Ser-520 relative to the total amount of DYRK1A over time from the initiation of bFGF stimulation. Unstimulated cells were assigned the baseline value of 1 in each experiment. Data correspond to means ± SEM from four independent experiments. *p ≤ 0.05; **p ≤ 0.001.