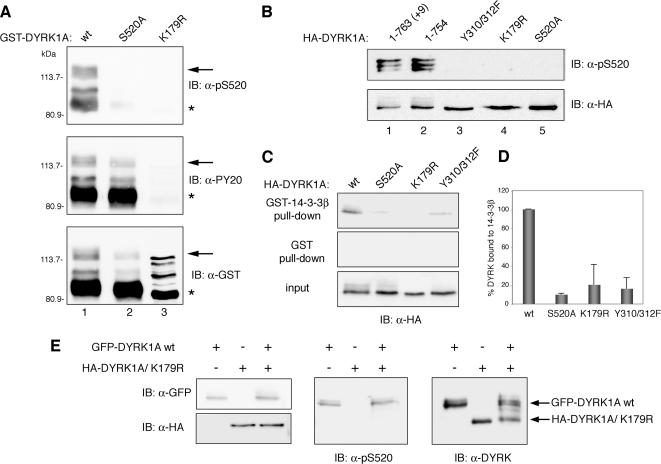
Figure 4.
DYRK1A autophosphorylates Ser-520 through an intramolecular reaction. (A) Bacterially expressed GST fusion proteins of wild-type DYRK1A (wt, lane 1) and the mutants DYRK1A/S520A (lane 2) and DYRK1A/K179R (lane 3) were immunoblotted with anti-DYRK1A pS520 (top), anti-phospho-Tyr (PY20; middle), and anti-GST (bottom) antibodies. The positions of marker proteins (in kilodaltons) are indicated. Arrows indicate full-length GST-fusion proteins, and asterisks indicate truncated GST-fusion proteins lacking the C-terminal (∼150) amino acids). The different patterns in DYRK1Awt, DYRK1AS520A, and DYRK1AK179R detected with the anti-GST antibody are due to differences in phosphorylation levels as shown in Supplemental Figure S3, A. (B) HA-tagged proteins corresponding to the two major isoforms of DYRK1A (lanes 1 and 2) and to distinct catalytically inactive point mutants (lanes 3–5) were overexpressed by transient transfection into U2-OS cells. Phosphorylation of Ser-520 was detected by immunoblotting with the phospho-specific anti-DYRK1A pS520 antibody (top). To control for equal expression of the fusion proteins, membranes were stripped and reprobed with an anti-HA antibody (bottom). (C) U2-OS cells were transfected with pHA-DYRK1Awt, pHA-DYRK1A/S520A, pHA-DYRK1A/K179R, or pHA-DYRK1A/Y310,312F, and soluble lysate fractions were incubated with unfused GST or GST-14-3-3β. Samples were analyzed by SDS-PAGE followed by immunoblotting with anti-HA antibody. Lysate represents 10% of input. (D) Quantification of the relative binding of different DYRK1A mutants to 14-3-3β compared with the fraction bound to DYRK1Awt is shown in the chart, which represents means ± SEM from three independent experiments. (E) A GFP-DYRK1A wild-type fusion protein (GFP-DYRK1Awt) and an HA-tagged DYRK1A kinase-inactive mutant (HA-DYRK1A/K179R) were individually expressed or coexpressed in U2-OS cells and detected by immunoblotting with anti-GFP and anti-HA antibodies, respectively (left). Phosphorylation of Ser-520 was analyzed by immunoblotting with the anti-DYRK1A pS520 antibody (middle). The expression levels of the two DYRK1A fusion proteins were compared by immunoblotting with anti-DYRK1A antibody (right).