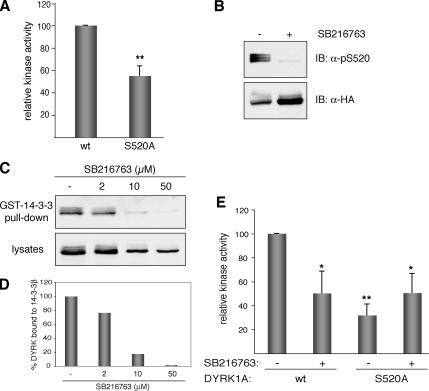
Figure 5.
Kinase activity of DYRK1A is modulated by the phosphorylation state of Ser-520. (A) US-OS cells were transfected with pHA-DYRK1Awt and the mutant pHA-DYRK1A/S520A. To determine the kinase activity of DYRK1A, tagged proteins were isolated by immunoprecipitation with anti-HA antibody, and immunocomplexes were subjected to in vitro kinase assay with DYRKtide as substrate. Equal amounts of HA-tagged proteins were used in all cases. The data represent means ± SEM from five independent experiments. **p ≤ 0.001. (B) U2-OS cells expressing HA-DYRK1Awt were treated during 8 h with the inhibitor SB216763 (50 μM) or with 0.1% DMSO as vehicle-control. Whole-cell extracts were resolved by 8% SDS-PAGE and immunoblotted with the anti-DYRK1A pS520 antibody (top). The membrane was then stripped and reprobed with anti-HA antibody to detect total HA-DYRK1A protein levels (bottom). (C) U2-OS cells expressing HA-DYRK1Awt were treated with increasing doses of SB216763, and soluble extracts were incubated with recombinant GST-14-3-3β. The amounts of bound DYRK1A (top) and DYRK1A in lysates (10% of inputs) (bottom) in each condition were analyzed by immunoblotting with anti-HA antibody. (D) The chart shows the quantification of the results expressed as the ratio of bound protein to input for each inhibitor concentration. (E) Kinase activities were determined as described in A for HA-DYRK1Awt and HA-DYRK1A/S520A expressed in U2-OS cells treated with 50 μM SB216763 for 8 h. For nontreated cells DMSO was used as vehicle-control. The data represent means ± SEM from three independent experiments. *p ≤ 0.05; **p ≤ 0.001.