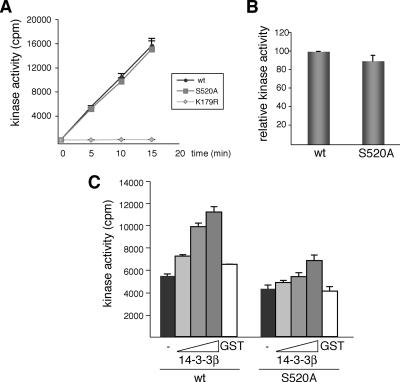
Figure 6.
14-3-3β binding to phospho-Ser-520 regulates DYRK1A kinase activity in vitro. (A) Kinase activity of recombinant DYRK1A proteins (GST-DYRK1Awt and the mutants S520A and K179R) was assayed with DYRKtide as substrate. The kinetics of the reaction indicated that 32P incorporation was linear with time and with the enzyme amount used in the assay. (B) The activity of GST-DYRK1Awt versus GST-DYRK1A/S520A is represented as the means ± SEM from three independent experiments, with each point measured in triplicate in the individual experiments. (C) Kinase activities of the indicated GST-DYRK1A proteins were assayed as described in A with increasing amounts of recombinant GST-14-3-3β (0.1, 0.5, and 1 μg) or GST alone (1 μg). The assay was performed three times, giving similar results in each; a representative assay is shown, and the data correspond to the means ± SEM of triplicate measurements.