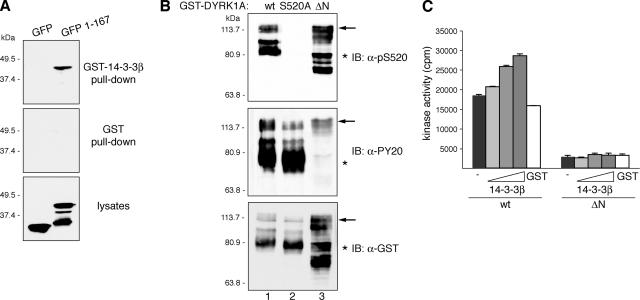
Figure 7.
DYRK1A harbors a second 14-3-3 binding site at the N terminus. (A) U2-OS cells were transfected with pEGFP (GFP) or pEGFP-DYRK1A/1-167 (1-167), and lysates were incubated with unfused GST or GST-14-3-3β. Samples were analyzed by immunoblotting with anti-GFP antibody. Lysates represent 10% of inputs. The positions of marker proteins (in kilodaltons) are indicated. (B) Bacterial fusion proteins corresponding to wild-type DYRK1A (wt; lane 1), the mutant DYRK1A/S520A (lane 2), and N-terminally truncated DYRK1A (ΔN; lane 3) were immunoblotted with anti-DYRK1A pS520 (top), anti-phospho-Tyr (PY20; middle), and anti-GST (bottom) antibodies. The positions of marker proteins (in kilodaltons) are indicated. Arrows indicate full-length GST-fusion proteins, and asterisks indicate truncated GST-fusion proteins lacking the C terminus. The abnormal gel mobility of the N-terminal–deleted mutant is explained by its hyperphosphorylation status as shown in Supplemental Figure S6. (C) Kinase activities of GST-DYRK1A proteins were assayed as described in Figure 6C, but GST-DYRK1Awt was compared with the N-terminally truncated mutant GST-DYRK1A/ΔN.